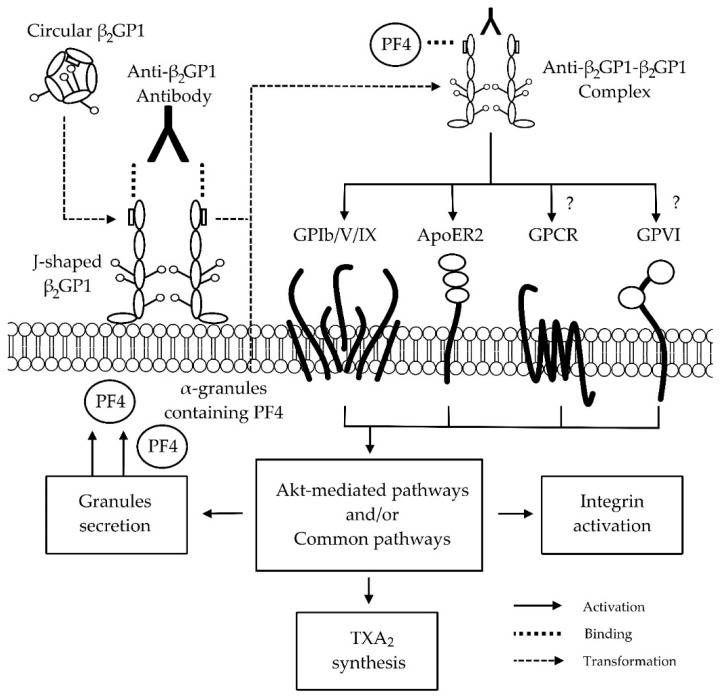
Figure 2.
Proposed mechanisms of interaction between anti-beta-2-glycoprotein 1 (β2GP1)-β2GP1 complex and platelet receptors. Circular β2GP1 binds to the anionic phospholipid platelet membrane and transforms into J-shaped β2GP1. This allows the anti-domain 1-β2GP1 antibody to bind and to form the anti-β2GP1-β2GP1 complex. The anti-β2GP1-β2GP1 complex has been proposed to interact with glycoprotein (GP) Ib of GPIb/V/IX [14] and apolipoprotein E receptor 2 (ApoER2) [96,97,98]. In our group, we propose that the complex might trigger adenosine diphosphate (ADP) and collagen-mediated pathways via guanine nucleotide-binding protein coupled receptor (GPCR) and GPVI, respectively [99,100]. Yet, further studies are needed to clarify the variability of results. The binding of the complex with receptors leads to the activation of protein kinase B (Akt)-mediated and/or common pathways, causing granules secretion, thromboxane A2 (TXA2) synthesis, integrin activation, and subsequently, clot formation. The platelet factor 4 (PF4) from secreted α-granules have also been showed to interact with the anti-β2GP1-β2GP1 complex [101] Abbreviations: β2GP1, beta-2-glycoprotein 1; GP, glycoprotein; ApoER2, apolipoprotein E receptor 2; ADP, adenosine diphosphate; GPCR, guanine nucleotide-binding protein coupled receptor; TXA2, thromboxane A2; PF4, platelet factor 4; Akt, protein kinase B.