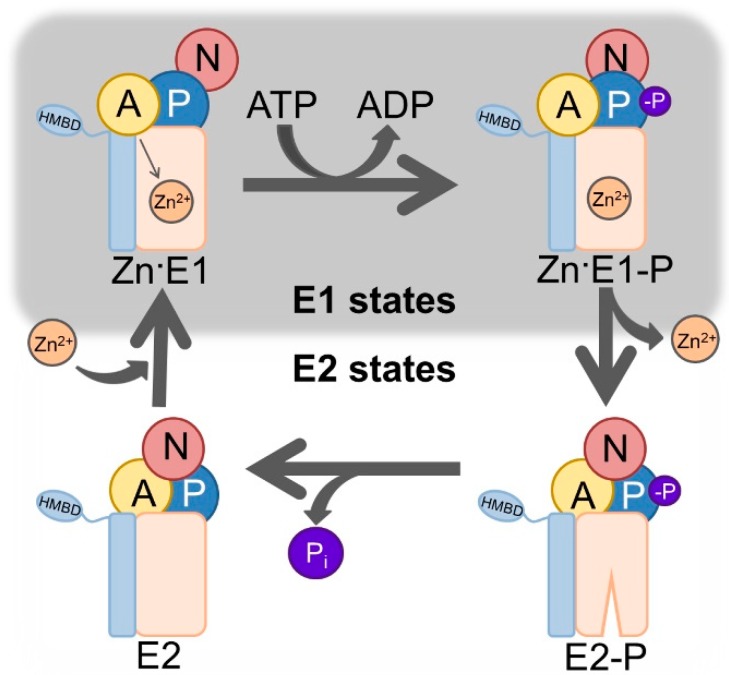
Figure 1.
Post-Albers scheme of PIB-2-ATPases. The E1 (high zinc affinity) and E2 (low zinc affinity) states of the enzyme alternate, and couple ATP (adenosine triphosphate) hydrolysis to the export of zinc. The E1 state accepts one zinc (Zn2+) ion and ATP from the intracellular side, which promotes autophosphorylation, reaching the zinc occluded Zn·E1-P state and releasing ADP (adenosine diphosphate). Completion of phosphorylation triggers considerable conformational changes that opens the pump towards the outside, allowing release of zinc in the E2-P state. Metal discharge is associated with auto dephosphorylation, liberation of inorganic phosphate (Pi), and allows the enzyme to reach the E2 conformation. The domains are represented as follows: The actuator (A) domain in yellow, the phosphorylation (P) domain in blue, the nucleotide-binding (N) domain in red, the transmembrane domain in light orange. Features specific for PIB-ATPases are shown in light blue, and includes two transmembrane helices and heavy-metal binding domain(s) (HMBD).