Figure 1.
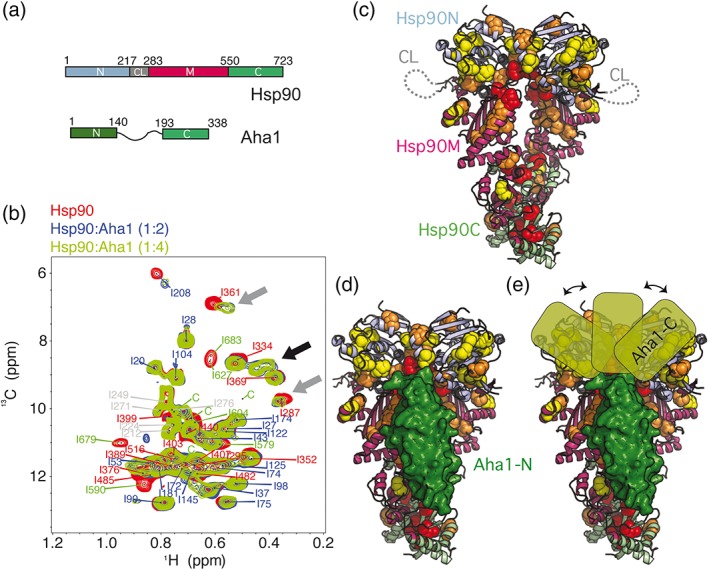
Interaction of Aha1 with human Hsp90 in the absence of nucleotide. (a) Domain organization of human Hsp90β and Aha1. The N‐terminal domain (Hsp90N) contains the ATPase active site, and Hsp90C is responsible for dimerization. The domain color code is kept in all figures. (b) Aha1 binding promotes strong changes in the methyl‐TROSY NMR spectra of Hsp90, including chemical shift perturbations and line broadening of Hsp90 isoleucine moieties (gray arrows) and the appearance of new signals (black arrow). The sequence‐specific assignment of cross‐peaks is indicated (Table S1). Four unassigned Hsp90C cross‐peaks are labeled with “c.” (c) Distribution of broadened Hsp90 isoleucine residues in Aha1 interaction and allostery on the structure of the closed Hsp90 dimer (PDB id 5fwk).19 Red, orange, and yellow spheres represent NMR perturbations of decreasing magnitude (shown in Figure S1b). The flexible charged linker of Hsp90 is represented by a dotted line. (d) Location of Aha1‐N (in green surface representation) on the structure of Hsp90 (PDB id 1usu).20 (e) The Aha1‐affected regions of Hsp90N suggest multiple bound conformations of the C‐terminal domain of Aha1 (Aha1‐C)
