Figure 3.
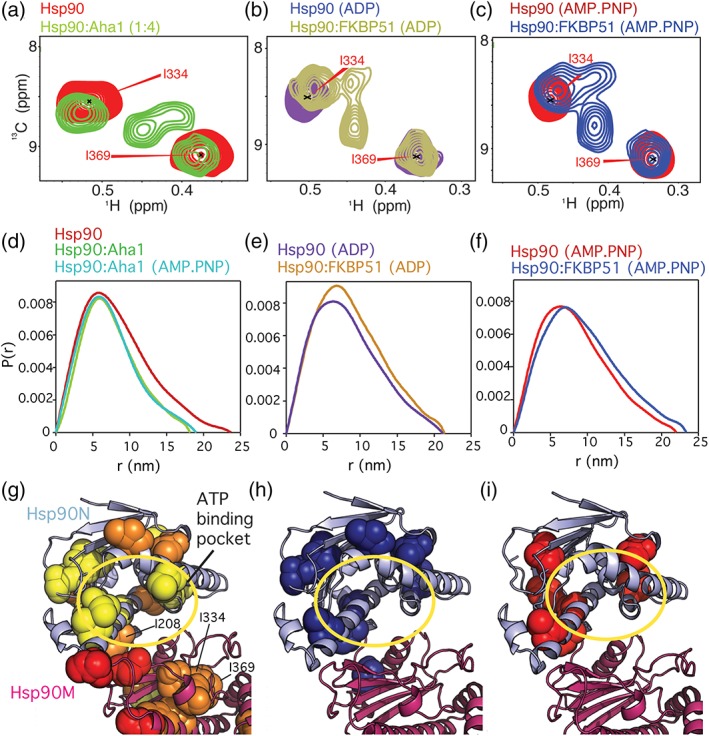
Structural changes induced by Aha1 and nucleotide binding. (a) New signals appear upon Aha1‐binding in the methyl‐TROSY spectra of full‐length Hsp90 in the absence of nucleotide. (b, c) New cross peaks appear upon binding of nucleotide and FKBP51. These signals are similar for ADP and AMP.PNP but are partially different from the peaks appearing in the presence of Aha1 (a). (d) SAXS P(r) distribution shows that Aha1 promotes a partially closed conformation13, 15 of Hsp90 independent of nucleotide. (e, f) FKBP51 stabilizes the extended conformation of Hsp90.7 (g) Aha1 promotes structural changes around the ATP‐binding pocket in Hsp90N in the absence of nucleotide. (h, i) Nucleotide binding promotes structural rearrangements around the ATP‐binding pocket.7 Spheres in (g–i) represent isoleucine residues affected in the corresponding binding process. A yellow circle in (g–i) highlights the ATP‐binding pocket
