Abstract
Background:
Single-event multilevel surgery (SEMLS) approach is regarded as the golden standard in developed countries to improve gait and functional mobility in children with cerebral palsy (CP). However, this approach is not always feasible in developing countries. Therefore, orthopedic surgery based on an interval surgery approach (ISA) is still commonly used in developing countries, although little is known about the long term outcomes of an ISA. Therefore, the aim of this study was to describe the gait patterns of adults with CP, who have been treated with ISA, which started more than 15 years ago.
Materials and Methods:
Thirty adults with CP and spastic diplegia, who received ISA treatment 21.6–33.7 years ago, were recruited for this study and participated in three-dimensional gait analysis. Twenty kinematic and nondimensional temporal-distance parameters were captured, while the overall gait deviation index (GDI) was also calculated. Data of the adults with CP were compared to normative data of typically developing (TD) adults.
Results:
Although all adults with CP were still ambulant, their gait parameters significantly differed from TD adults, with a lower GDI in the adults with CP. The CP gait patterns were characterized by excessive hip flexion and hip internal rotation as well as a stiff-knee gait.
Conclusion:
Although different to TD adults, the gait patterns observed in the adult with CP treated with ISA is in line with other studies. Gait patterns suggest that derotation osteotomies potentially could have improved the long term gait patterns. Although SEMLS might be the preferred treatment method, potentially resulting in better outcomes, ISA can also be used to treat children with CP in developing countries as India and South Africa, where a SEMLS approach is not always feasible.
Keywords: Adults, cerebral palsy, gait, interval surgery approach, long term followup, orthopedic surgery
Introduction
Cerebral palsy (CP) is described as a nonprogressive permanent disorder of the immature brain, which typically results in a wide range of secondary musculoskeletal abnormalities in childhood.1 Orthopedic interventions are often performed to prevent the development of contractures and/or lever arm dysfunction.2,3 One of the main outcome goals of these surgeries is maintaining a functional walking pattern which allows people with CP to remain independent.4
In order to assess the effectiveness of different surgical interventions, more and more studies are using three-dimensional gait analysis (3DGA) as a tool to measure the impact of surgical interventions on walking patterns.5,6,7,8 A recent study by McGinley et al.,5 systematically reviewed the effects of single-event multilevel surgery (SEMLS) on gait patterns for up to 5 years after surgery. This study reported that kinematic gait parameters in 89% (16/18) and temporal distance gait parameters in 53% (8/15) of the cases improved after the SEMLS intervention. Although maturation and growth also affect the development of gait patterns up to the age of 7 years,2,9 these findings suggest that SEMLS is highly effective in the creation and/or improvement of a healthy gait pattern in children with CP.5
Before the introduction of SEMLS, which is defined as two or more soft tissue or bony surgical procedures at two or more anatomical levels during one surgical intervention,5 children were generally treated on multiple occasions. This is commonly known as the “birthday syndrome” approach,10 or even better defined as the “interval surgery approach” (ISA) since the children are not operated annually. Although SEMLS is widely regarded as the standard of care in most developed countries, ISA is more commonly used in developing countries where it is not always feasible to use a SEMLS approach.
Despite the fact that ISA has been around for many decades and still performed in developing countries, no followup studies are available of adults with CP, who underwent these orthopedic interventions in childhood. Information about the gait patterns of adults with CP who underwent ISA will be highly valuable in: determining how mobile these adults still are, creating comparative data to be used in future studies regarding the additional benefits of a SEMLS approach on gait, and providing clinicians and patients with a reference as to what type of gait pattern can be expected based on an ISA.
Therefore, the aim of the study was to describe the gait pattern (based on the 3DGA kinematics, nondimensional temporal distance parameters, and gait deviation index [GDI]) in adults with CP, who received the first orthopedic surgery based on ISA more than 15 years ago, and compare this with typically developing (TD) adults.
Materials and Methods
This study was approved by the Human Research Ethics Committee at the University of Cape Town and conducted in accordance to the principles of the Declaration of Helsinki. Each participant provided written informed consent upon gaining satisfactory knowledge regarding the risks and benefits of participation.
The participants were recruited from the database of a special need school for disabled children in Cape Town, South Africa. The criteria for inclusion stated that participants had to have been born prematurely and diagnosed with CP and spasticity in both lower limbs (could have mild involvement of an upper limb unilaterally). In addition, participants had to be ambulant at the time of research, with or without the aid of an assistive device. Participants were also required to have been ambulatory by the age 4 of years (with or without walking and/or support). In addition, they should have received initial orthopedic surgery 15 years previously with the goal to improve function and gait (if the child was <4 years and not ambulant preoperatively, the goal was to develop locomotion). The participants also had to have received standard care, including conservative treatment (e.g., orthosis, casting, and physiotherapy sessions at a frequency which had been individually indicated). Preoperative clinical records from the physiotherapists at the school had to be available, including information about the child's physical (e.g., muscle tone, joint mobility, and motor control) and functional (e.g., able to sit, stand, walk, run, and jump) status as well as the use of assistive devices and interventions received. Finally, for followup purposes, the participant had to live within a 100 km range of the city of Cape Town. Persons were excluded if they had been diagnosed with dystonia, athetosis, ataxia, hypotonia, and other neuromuscular disorders. Persons were also not accepted in the study if they had undergone a neurosurgical intervention, such as selective dorsal rhizotomy (SDR), in the past.
As mentioned before, eligible participants were screened on the basis of physiotherapy clinical records, which were obtained from the school. Of the 50 eligible participants, 30 consented to participate in this study, while 13 participants were untraceable and 7 patients did not want to participate due to reasons that were not health related.
Participants were interviewed using a structured questionnaire detailing demographic characteristics and surgical information. Details of orthopedic interventions received, related to CP, were obtained from clinical reports and verified by examining scars and/or questioning parents or caretakers where necessary. These detailed reports were also used to retrospectively determine the preoperative gross motor function classification system (GMFCS) levels.11,12 In addition, body mass index (BMI) was calculated, and the socioeconomic status (SES) of participants was determined by housing density. This SES indicator refers to the number of people living in a house, divided by the number of rooms, excluding the kitchen and bathroom.13 A high SES is considered a housing density ratio of <1.0 and a low SES refers to a ratio of >1.5.
Gait data were established through the collection of marker trajectories recorded with an 8-camera motion capture system (Vicon MX T-series, Vicon Ltd, Vicon UK) operating at 240 Hz. Sixteen reflective markers were used as prescribed for the lower body Plug-in-Gait marker placement and model. Participants were asked to walk at a self-selected comfortable speed, wearing their usual footwear, along with a 15 m walkway. The participants were allowed the use of assistive device(s) if this was normally also used in their everyday life. At least, five trials were captured, from which three trials that were deemed to be of good quality (visible markers, typical speed/pattern as judged by observers) were selected for data processing.
The normal distribution of the gait data was determined through the Kolmogorov–Smirnov and Lilliefors test and subsequently reported as mean and standard deviations. Gait graphs of the pelvis, hip, knee, and ankle were created for the frontal, sagittal, and transverse planes. These data were presented together with graphs based on normative data from typical developed adults, who had no history of orthopedic or neurological pathology.14 In addition, 20 kinematic gait parameters were calculated including – pelvis: mean tilt, range of motion (ROM) in sagittal, and transverse planes; hip: maximal extension and adduction, mean rotation, ROM in sagittal, and frontal planes; knee: initial contact (IC) flexion, ROM in sagittal plane, maximal extension, and flexion; ankle: IC and mean dorsi/plantar flexion, maximal plantarflexion and dorsiflexion, and mean foot progression. In addition, nondimensional temporal distance parameters were also reported and included: time to foot off, speed, and cadence.15 The GDI, which quantified the gait pattern in one score16 and reported satisfactory face validity in adults with spastic CP17 was also calculated.
Differences between gait parameters and the GDI were analyzed between the adults with CP and the TD adults, with independent sample t-tests, as also previously described.14 Statistical significance was accepted at P < 0.05.
Results
The 30 participants, 12 males and 18 females, who enrolled in the study, had a median age of 32 years (range 18–46 years). At the time of the first surgery, the participants were between 2 and 12 years of age, but 22 participants (73%) were younger than 7 years. This resulted in a followup time of 15–39 years, with 7 (23%) being seen between 15 and 20 years, 11 (37%) between 21 and 30 years and 12 (40%) 31–40 years after the first orthopedic intervention. The BMI of the participants varied considerably, ranging from 18 to 40, with 14 participants (46%) being overweight. The SES was almost equally distributed within the categories, with a wide range of housing density ratio of 0.5–5.0 [Table 1].
Table 1.
Demographic and personal background information (n=30)
| Personal and clinical characteristics | Median (IQR)/n (%) |
|---|---|
| Age, median (IQR) | |
| At initial intervention (years) | 4.6 (3.6-7.0) |
| Current (years) | 32.8 (28.6-39.3) |
| Followup time, median (IQR) | |
| Between initial intervention and current (years) | 27.7 (22.1-33.4) |
| Orthopaedic interventions, median (IQR) | |
| Total number of interventions | 5 (2-6) |
| Number of events | 3 (2-4) |
| Gender, n (%) | |
| Female | 18 (60) |
| Male | 12 (40) |
| BMI, n (%) | |
| Underweight | 4 (13) |
| Normal | 12 (40) |
| Overweight | 7 (23) |
| Obese | 7 (23) |
| SES, n (%) | |
| Low | 10 (33) |
| Middle | 11 (37) |
| High | 9 (30) |
IQR=Interquartile range, SES=Socioeconomic status, BMI=Body mass index
Functional status (walking ability) preoperatively varied from walks without limitation (GMFCS level I) to walks using a handheld mobility device (GMFCS level III). Preoperative presentation of the GMFCS scores was: 9 participants (30%) at level I, 14 participants (47%) at level II, and 7 participants (23%) at level III. Postoperatively, 15 adults (50%) were classified as GMFCS I, 11 adults (37%) were classified as GMFCS II, and 4 adults (13%) were classified as GMFCS III.
As defined by ISA, the participants receiving multiple interventions (referring to number of surgical procedures at different events). The study cohort reported a median of 5 interventions (range 1–11) with a median of 3 events (range 1–7) as shown in Table 1. The most prevalent interventions and revisions were on the Achilles tendon and Hamstrings. Twenty-eight adults with CP received Achilles tendon surgery (93%), with eight adults receiving one revision, one adult receiving two revisions, and one adult receiving three revisions. Hamstring interventions were performed on 17 adults (57%), with three adults receiving one revision and one adult receiving four revisions. Lever-arm dysfunction surgery was less commonly seen, with only 5 adults (17%) who had received femoral and/or tibial derotation osteotomy surgery [Table 2].
Table 2.
Overview of the number of participants received soft tissue and/or bony surgery at lower extremities, with a specification of repetitions performed
| Orthopaedic intervention | One intervention, n | One repetition, n | Two or more repetitions, n | Total received the intervention, n (%) |
|---|---|---|---|---|
| Soft-tissue surgery | ||||
| Achilles tendon | 18 | 8 | 2* | 28 (93) |
| Gastrocnemius (vulpius) | 6 | 1 | 7 (23) | |
| Rectus femoris | 8 | 1 | 9 (30) | |
| Hamstrings | 13 | 3 | 1** | 17 (57) |
| Adductors | 10 | 1 | 11 (37) | |
| Psoas | 6 | 6 (20) | ||
| Abductor hallucis longus | 3 | 3 (10) | ||
| Tibialis posterior | 2 | 1 | 3 (10) | |
| Peroneus | 2 | 1 | 3 (10) | |
| Bony surgery | ||||
| Femoral derotation | 5 | 5 (17) | ||
| Tibial derotation | 3 | 1 | 4 (13) | |
| Ankle/foot corrections | 9 | 1 | 1* | 11 (37) |
| Toe corrections | 0 | 1 | 1 (3) |
*3 repetitions per orthopedic intervention, **4 repetitions per orthopedic intervention
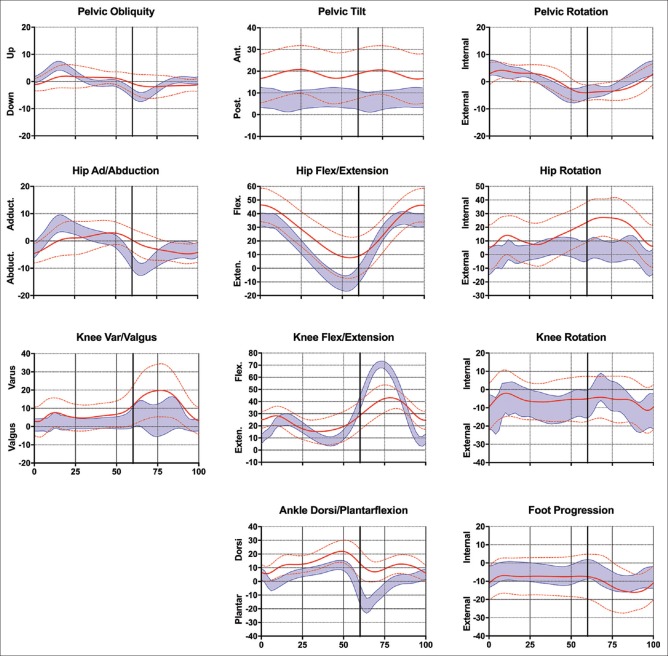
Deviations in the gait pattern were mostly detected in the sagittal plane with an increased anterior pelvic tilt throughout the gait cycle, decreased peak hip extension and associated decreased hip flexion/extension ROM, reduced peak knee flexion and extension resulting in a decreased knee flexion ROM and a more dorsiflexed pattern for the ankle with reduced peak plantar and increased dorsal flexion throughout the gait cycle. A reduced hip adduction/abduction ROM and an increased hip internal rotation throughout the gait cycle was also noted. Interestingly, mean foot progression did not differ to the normative data [Figure 1 and Table 3].
Figure 1.
Kinematic data; mean ± standard deviation for the adults with cerebral palsy (red lines) and normative data of typically developing adults (n = 43) (blue band)
Table 3.
Overview of kinematic and temporal distance gait parameters of adults with cerebral palsy and indication of significant differences with normative data
| Parameters | Adult CP group | Normative data14 | P | ||||
|---|---|---|---|---|---|---|---|
| Mean±SD | Min | Max | Mean±SD | Min | Max | ||
| Pelvis (°) | |||||||
| Mean tilt | 18.7±11.4 | −4.5 | 38.3 | 7.2±4.4 | 0.4 | 19.2 | <0.01* |
| ROM tilt | 9.5±4.0 | 2.4 | 20.2 | 3.2±1.1 | 1.6 | 7.0 | <0.01* |
| ROM rotation | 15.1±4.7 | 7.8 | 29.1 | 11.7±4.8 | 3.3 | 28.1 | <0.01* |
| Hip (°) | |||||||
| Max extension | 6.9±14.9 | −12.1 | 37.8 | −11.4±5.7 | −21.8 | 3.5 | <0.01* |
| ROM flexion/extension | 41.2±8.4 | 23.8 | 60.1 | 49.2±3.2 | 42.5 | 55.8 | <0.01* |
| Max adduction | 5.9±3.0 | −2.1 | 14.4 | 6.8±3.0 | 0.5 | 14.0 | 0.25 |
| ROM adduction/abduction | 14.1±3.7 | 8.9 | 22.5 | 17.6±3.7 | 10 | 27.0 | <0.01* |
| Mean rotation | 16.0±14.1 | −19.2 | 40.8 | 2.1±6.7 | −12.9 | 13.1 | <0.01* |
| Knee (°) | |||||||
| IC flexion | 25.0±7.0 | 12.3 | 37.7 | 9.0±3.7 | −1.0 | 15.6 | <0.01* |
| Max extension | 12.6±10.9 | −21.5 | 31.2 | 5.2±3.2 | −3.8 | 11.5 | <0.01* |
| Max flexion | 46.7±8.0 | 24.9 | 62.6 | 70.9±2.8 | 65.4 | 76.8 | <0.01* |
| ROM flexion/extension | 34.1±10.8 | 16.4 | 52.1 | 65.7±3.4 | 58.7 | 72.5 | <0.01* |
| Ankle/foot (°) | |||||||
| Mean dorsi/plantarflexion | 12.6±6.0 | 0.8 | 24.8 | 1.7±2.7 | −3.9 | 7.1 | <0.01* |
| Max plantarflexion | 1.0±5.7 | −8.2 | 14.0 | −18.9±5.2 | −32.3 | −10.5 | <0.01* |
| Max dorsiflexion | 24.6±7.3 | 10.3 | 40.4 | 12.6±3.0 | 5.7 | 19.2 | <0.01* |
| IC dorsi/plantarflexion | 6.7±5.8 | −4.4 | 19.2 | 5.4±4.0 | −4.3 | 15.2 | 0.29 |
| Mean foot progression | −9.6±10.2 | −32.7 | 20.5 | −6.8±4.6 | −19.3 | 0.8 | 0.12 |
| Temporal distance parameters | |||||||
| ND speed | 0.30±0.10 | 0.10 | 0.47 | 0.48±0.04 | 0.36 | 0.60 | <0.01* |
| ND cadence | 0.51±0.09 | 0.32 | 0.65 | 0.58±0.03 | 0.49 | 0.63 | 0.01* |
| Time to foot off (%) | 61.3±5.1 | 46.2 | 72.5 | 58.0±1.6 | 54.9 | 63.2 | <0.01* |
SD=Standard deviation, Min=Minimum value, Max=Maximum value, P=P value, ROM=Range of motion, IC=Initial contact, ND=Nondimensional, CP=Cerebral palsy. *P < 0.05 (significantly different)
Nondimensional gait parameters, speed, and cadence were different compared to the normative data. Adults with CP walked at a decreased speed and cadence and exhibited a longer stance phase (time to foot off) as shown in Table 3.
The distribution of the GDI scores showed moderate residual gait deviations, with a mean GDI score in the adults with CP of 68.2 ± 14.0, while the mean GDI score for the TD adults was 100.0 ± 10.0, as expected.16
Discussion
The main aim of this study was to describe the gait pattern of adults with CP, who received multiple orthopedic interventions at different points of time during childhood (ISA), of which the first intervention had been performed more than 15 years ago. The median time after the initial orthopedic intervention was 27 years with adults having received up to 11 surgeries (median of 3 surgeries).
The first finding of this study was that all adults with CP were still ambulant and could be classified as either GMFCS level I (50%), II (37%), or III (13%). Interestingly, 40% of the adults displayed a lower GMFCS level (thus better) than preoperative, 50% were unchanged, while 10% had a higher GMFCS level (deterioration). In the developed countries, the GMFCS levels are believed to remain unchanged after a surgical intervention.18 This is supported by Alriksson-Schmidt et al.19 who reported, based on a study conducted in Sweden, no change in 74% of a large CP cohort (n = 736) 20 years after surgery. In contrast to developed countries, changes in GMFCS levels are frequently noted in developing countries, as also confirmed in the current study conducted in South Africa. This can possibly be contributed to the nonaccess to presurgical treatment opportunities in developing countries. As also seen in our cohort, nonsurgical treatment options, as botulinum toxin injections, were not available to our cohort, which is common practice within African institutions.20,21 This absence is likely to result in the relatively larger postsurgical changes in GMFCS levels, than in children who do receive nonsurgical treatment intervention. This difference might explain why permanent changes in GMFCS are frequently seen in developing countries, while it is a rare in developed countries.
The main finding of this study was that all adults with CP still had a functional gait pattern. However, gait parameters were significantly different to that of their age-matched typically developed peers [Table 3]. Overall, the adults with CP walked with a flexed gait pattern, based on increased anterior pelvic tilt, hip, and knee flexion, and dorsiflexion throughout the gait cycle [Figure 1].
Looking in more detail, certain specific gait abnormalities as described by Wren et al.22 could be determined. In the sagittal plane, excessive hip flexion and a stiff knee gait pattern were seen. Excessive hip flexion can be defined as “Hip flexed more than 0 in terminal stance,” while Stiff Knee gait is defined as “Decreased arc of knee motion from maximum knee extension in stance to peak knee flexion in swing, and/or delay in peak swing knee flexion to mid or terminal swing, hindering foot clearance.” Both gait abnormalities are common in people aging with CP and spastic diplegia, with a prevalence of 68% for excessive hip flexion and 94% for stiff knee gait in people who had previous surgeries. In the transverse plane, excessive internal hip rotation was seen, which can be defined as “Internal hip rotation more than one standard deviation more than mean for normal for significant portion of stance.” Wren et al.22 also described this as a common abnormality, with a prevalence of 49% in the aging CP population with spastic diplegia, who underwent surgery in the past. Herewith, they highlighted that this was often a result of ignorance of addressing bony rotational problems in childhood. This could also be the reason for the rotational malalignment seen in the current study, with osteotomies performed in <20% of the current cohort. As shown in a systematic review,6 lever-arm dysfunction surgeries have besides a positive effect on the gait pattern in the transverse plane, also improve sagittal plane hip, knee, and ankle kinematics. As this type of orthopedic surgery is much more common today, the gait pattern of patients receiving osteotomies might be better than what was reported in the current study.
The gait pattern of adults with CP after ISA was compared to that of TD peers; however, the question can be posed whether this is fair. It would have a preference to compare to adults with the same diagnosis, who received other interventions. In line with this, Langerak et al.,14 studied 32 adults with CP and spastic diplegia, who underwent the neurosurgical procedure SDR more than 15 years previously. The authors noted similar gait patterns to those reported in the current study, except for the slope of maximum knee flexion angle in late stance and early swing phase (SDR study no Stiff Knee gait). Future research could determine whether these differences are indeed significant and whether they can be ascribed to difference in, for example, muscle tone since this was specifically addressed in SDR, while ISA focused on treating soft-tissues (e.g., tendon lengthening) and bony abnormalities (e.g., osteotomies).
Although orthopedic surgery techniques have significantly changed over the last few decades5,6 and SEMLS interventions have become the preferred treatment approach, this research study provides novel and clinical insights into the gait pattern of adults with CP in the long term after ISA. This knowledge is important especially to clinicians and researcher who work in countries, such as South Africa and India, where not all children with CP can be treated with a SEMLS approach.
Conclusion
Adults with CP and spastic diplegia, who received multiple interventions on an interval basis (ISA) more than 15 years ago, were still ambulant but had a more flexed gait pattern than their typically developed peers. The observed gait pattern was characterized by stiff knee gait, excessive hip flexion and hip internal rotation, and a slower walking speed. Although SEMLS nowadays is the preferred approach to treat children with CP, this study shows also good functional outcome results in children who have been treated with ISA. These findings are of clinical and scientific importance for developing countries, such as India and South Africa, where a SEMLS approach is not always feasible. In addition, the study also provides unique insight on how ISA can further be optimized in the future (e.g., the potential value of including de-rotation osteotomies) to gain even better outcomes for adults with CP and spastic diplegia who need orthopedic surgical interventions.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
The study was funded by the National Research Foundation of South Africa.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank all who participated in this study. In addition, we would like to extend our gratitude to the South African National Research Foundation (NRF) for their financial support.
References
- 1.Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, et al. A report: The definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109:8–14. [PubMed] [Google Scholar]
- 2.Narayanan UG. Management of children with ambulatory cerebral palsy: An evidence-based review. J Pediatr Orthop. 2012;32(Suppl 2):S172–81. doi: 10.1097/BPO.0b013e31825eb2a6. [DOI] [PubMed] [Google Scholar]
- 3.Novak I, McIntyre S, Morgan C, Campbell L, Dark L, Morton N, et al. A systematic review of interventions for children with cerebral palsy: State of the evidence. Dev Med Child Neurol. 2013;55:885–910. doi: 10.1111/dmcn.12246. [DOI] [PubMed] [Google Scholar]
- 4.Morgan P, McGinley J. Gait function and decline in adults with cerebral palsy: A systematic review. Disabil Rehabil. 2014;36:1–9. doi: 10.3109/09638288.2013.775359. [DOI] [PubMed] [Google Scholar]
- 5.McGinley JL, Dobson F, Ganeshalingam R, Shore BJ, Rutz E, Graham HK. Single-event multilevel surgery for children with cerebral palsy: A systematic review. Dev Med Child Neurol. 2012;54:117–28. doi: 10.1111/j.1469-8749.2011.04143.x. [DOI] [PubMed] [Google Scholar]
- 6.Lamberts RP, Burger M, du Toit J, Langerak NG. A systematic review of the effects of single-event multilevel surgery on gait parameters in children with spastic cerebral palsy. PLoS One. 2016;11:e0164686. doi: 10.1371/journal.pone.0164686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson DJ, Scully WF, Min KS, Harmon TA, Eichinger JK, Arrington ED. Biomechanical analysis of intramedullary vs. superior plate fixation of transverse midshaft clavicle fractures. J Shoulder Elbow Surg. 2016;25:949–53. doi: 10.1016/j.jse.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Wilson NC, Chong J, Mackey AH, Stott NS. Reported outcomes of lower limb orthopaedic surgery in children and adolescents with cerebral palsy: A mapping review. Dev Med Child Neurol. 2014;56:808–14. doi: 10.1111/dmcn.12431. [DOI] [PubMed] [Google Scholar]
- 9.Hanna SE, Rosenbaum PL, Bartlett DJ, Palisano RJ, Walter SD, Avery L, et al. Stability and decline in gross motor function among children and youth with cerebral palsy aged 2 to 21 years. Dev Med Child Neurol. 2009;51:295–302. doi: 10.1111/j.1469-8749.2008.03196.x. [DOI] [PubMed] [Google Scholar]
- 10.Rang M. Cerebral Palsy. In: Morrissy R, editor. Lovell and Winter's Peadiatric Orthopaedics. Philadelphia: JB Lippincott Co.; 1990. pp. 465–506. [Google Scholar]
- 11.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–23. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 12.Palisano RJ, Rosenbaum P, Bartlett D, Livingston MH. Content validity of the expanded and revised gross motor function classification system. Dev Med Child Neurol. 2008;50:744–50. doi: 10.1111/j.1469-8749.2008.03089.x. [DOI] [PubMed] [Google Scholar]
- 13.Micklesfield LK, Levitt NS, Carstens MT, Dhansay MA, Norris SA, Lambert EV. Early life and current determinants of bone in South African children of mixed ancestral origin. Ann Hum Biol. 2007;34:647–55. doi: 10.1080/03014460701730024. [DOI] [PubMed] [Google Scholar]
- 14.Langerak NG, Tam N, Vaughan CL, Fieggen AG, Schwartz MH. Gait status 17-26 years after selective dorsal rhizotomy. Gait Posture. 2012;35:244–9. doi: 10.1016/j.gaitpost.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Hof AL. Scaling gait data to body size. Gait Posture. 1996;4:222–3. doi: 10.1016/s0966-6362(01)00097-2. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz MH, Rozumalski A. The gait deviation index: A new comprehensive index of gait pathology. Gait Posture. 2008;28:351–7. doi: 10.1016/j.gaitpost.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Maanum G, Jahnsen R, Stanghelle JK, Sandvik L, Larsen KL, Keller A. Face and construct validity of the gait deviation index in adults with spastic cerebral palsy. J Rehabil Med. 2012;44:272–5. doi: 10.2340/16501977-0930. [DOI] [PubMed] [Google Scholar]
- 18.Palisano RJ, Cameron D, Rosenbaum PL, Walter SD, Russell D. Stability of the gross motor function classification system. Dev Med Child Neurol. 2006;48:424–8. doi: 10.1017/S0012162206000934. [DOI] [PubMed] [Google Scholar]
- 19.Alriksson-Schmidt A, Nordmark E, Czuba T, Westbom L. Stability of the gross motor function classification system in children and adolescents with cerebral palsy: A retrospective cohort registry study. Dev Med Child Neurol. 2017;59:641–6. doi: 10.1111/dmcn.13385. [DOI] [PubMed] [Google Scholar]
- 20.Donald KA, Samia P, Kakooza-Mwesige A, Bearden D. Pediatric cerebral palsy in Africa: A systematic review. Semin Pediatr Neurol. 2014;21:30–5. doi: 10.1016/j.spen.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Donald KA, Kakooza AM, Wammanda RD, Mallewa M, Samia P, Babakir H, et al. Pediatric cerebral palsy in Africa: Where are we? J Child Neurol. 2015;30:963–71. doi: 10.1177/0883073814549245. [DOI] [PubMed] [Google Scholar]
- 22.Wren TA, Gorton GE, 3rd, Ounpuu S, Tucker CA. Efficacy of clinical gait analysis: A systematic review. Gait Posture. 2011;34:149–53. doi: 10.1016/j.gaitpost.2011.03.027. [DOI] [PubMed] [Google Scholar]