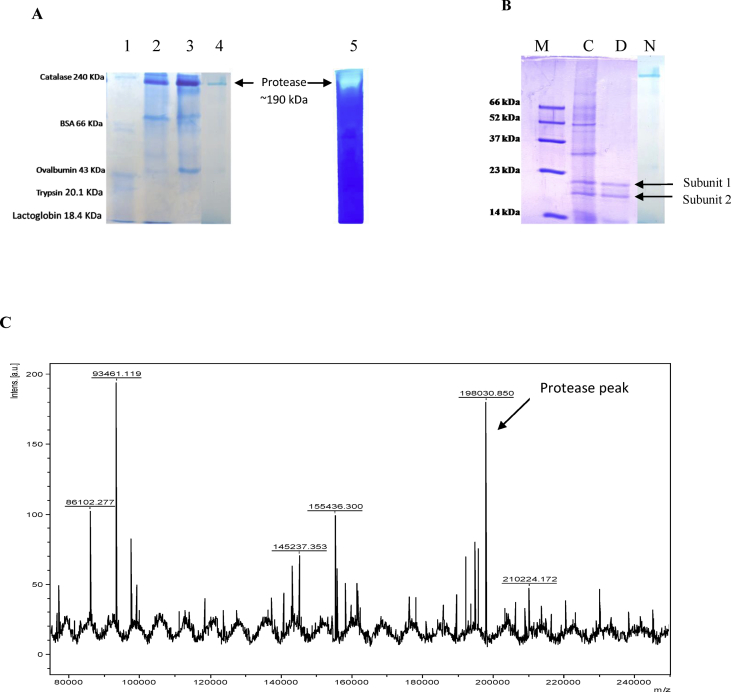
Fig. 3.
Electrophoretic profile of purified protease and molecular weight determination. (A) Native-PAGE analysis. Lane 1: standard protein marker; lane 2: enzyme after ammonium sulphate fractionation (0–90%); lane 3: protease fraction after ion exchange chromatography; lane 4: purified enzyme obtained after Sephadex G-200 chromatography; lane 5: zymogram of purified enzyme showing caseinolytic activity. (B) 12% SDS PAGE. M: standard marker; C: crude enzyme; D: purified enzyme run under denaturing conditions; N: purified enzyme run under non-denaturing conditions. (C) MALDI-TOF spectrum of purified enzyme. The mass spectrum shows a series of protonated molecular ions. The molecular mass of the enzyme was found to be 198030.850 Da.