Abstract
Many studies about classification and the functional annotation of intrinsically disordered proteins (IDPs) are based on either the occurrence of long disordered regions or the fraction of disordered residues in the sequence. Taking into account both criteria we separate the human proteome, taken as a case study, into three variants of proteins: i) ordered proteins (ORDPs), ii) structured proteins with intrinsically disordered regions (IDPRs), and iii) intrinsically disordered proteins (IDPs). The focus of this work is on the different functional roles of IDPs and IDPRs, which up until now have been generally considered as a whole. Previous studies assigned a large set of functional roles to the general category of IDPs. We show here that IDPs and IDPRs have non-overlapping functional spectra, play different roles in human diseases, and deserve to be treated as distinct categories of proteins. IDPs enrich only a few classes, functions, and processes: nucleic acid binding proteins, chromatin binding proteins, transcription factors, and developmental processes. In contrast, IDPRs are spread over several functional protein classes and GO annotations which they partly share with ORDPs. As regards to diseases, we observe that IDPs enrich only cancer-related proteins, at variance with previous results reporting that IDPs are widespread also in cardiovascular and neurodegenerative pathologies. Overall, the operational separation of IDPRs from IDPs is relevant towards correct estimates of the occurrence of intrinsically disordered proteins in genome-wide studies and in the understanding of the functional spectra associated to different flavors of protein disorder.
Introduction
Over the last two decades, the concept of ‘intrinsic disorder’ has emerged as a prominent and influential topic in protein science [1–7]. The discovery of intrinsically disordered proteins (IDPs), i.e., functional proteins lacking a well-defined tertiary structure, has challenged the traditional sequence-structure-function paradigm [8,9]. In their seminal paper, Uversky, Gillespie, and Fink [2] introduced the “charge-hydrophobicity phase space”, which clarified that IDPs are characterized by a combination of “low overall hydrophobicity and large net charge”. IDPs constitute, in eukaryotes, a substantial part of the cellular proteome and are involved in many biological processes that complement the functional repertoires of ordered proteins [10,11]. Ever-increasing experimental evidence has revealed the presence of disordered regions also in well-structured proteins [12,13]. Then the distinction between intrinsically disordered proteins, i.e., proteins lacking a tertiary structure, and structured proteins containing intrinsically disordered regions gradually emerged [14–17]. An operational distinction of these two variants is the main objective of the present work. A note on terminology is in order at this point. The editors of the journal Intrinsically Disordered Proteins made an effort to disambiguate the semantics of protein intrinsic disorder [18]. They proposed “intrinsically disordered proteins” as a unifying term, recognizing that it is a compromise “far from being ideal”, and suggesting that “additional descriptors” would emerge, apt to clarify the many aspects of “structurelessness” that are included in one term. The terminology we adopt here is surely also not ideal. Let us try to make clear that we use here IDPs, when referring to previous studies, as the general unifying term proposed by Dunker et al. We use IDPs also to denote a subgroup of Dunker et al.’s IDPs, a variant that is operationally specified here as distinct from the variant of mostly structured proteins with long intrinsically disordered regions (we call here IDPRs).
The biological role of IDPs has been the focus of a growing number of publications, in the recent past. Xie et al., for example, in a series of three articles compiled an anthology of the functional roles of IDPs [19–21]. It has been reported that out of the 710 keywords recording biological functions in the Uniprot/Swissprot database, 310 (44%) are associated with ordered proteins and 238 (34%) with IDPs [22]. Several studies have reported lists of Gene Ontology functional classes that are enriched in IDPs, including but not limited to: cell regulation, transcription, translation, signaling, and alternative splicing [23–30]. Different studies highlight the occurrence of unfolded regions in proteins that function as chaperones for other proteins or RNA molecules, transcription factors, effectors, assemblers, and scavengers [14–16,27]. Importantly, many proteins with disordered regions have been associated with human diseases [9,31,32], and a D2 concept (disorder in disorders) emerged [33]. However, in all these studies what we distinguish here as IDPs and IDPRs were considered as a unique variant, possibly aggregating proteins which are structurally and functionally different. This is still reflected, for example, in a recent paper by Darling and Uversky [34]. IDPRs, as we define them, may have a well-defined 3D structure that either can undergo conformational, allosteric changes (e.g., after a post-translational modification [35]) or accommodate functional, locally disordered domains, that are limited to less than 30% of the structure. The presence of unstructured loops or domains, e.g., linear motifs and Molecular Recognition Features (MORFs), can be important for these proteins to target low-affinity substrates enlarging the repertoire of protein interactions [15].
Let us remind that one of the features that distinguishes IDPs from structured proteins is their different interaction mechanism with target substrates. According to the classic lock-and-key mechanism [36], the specificity and affinity required for molecular interactions depend on the complementarity of the binding interfaces. However, even proteins that are disordered under physiological conditions or contain long unstructured regions can form stable complexes [9,37]. In this context, the lack of a well-defined 3D-structure represents a major functional advantage for IDPs, allowing them to interact with a broad range of substrates with relatively high-specificity and low-affinity, often undergoing a disorder-to-order transition upon binding [3,9,27,38,39]. However, despite that this description makes sense for proteins lacking a tertiary structure (IDPs), it is reasonable to suppose that structured proteins with long disordered regions (IDPRs) still interact with substrates through a mechanism similar to the lock-and-key. It has been reported that disordered regions have many post-translational modification sites (PTMs) [9,30,40–42], which induce conformational changes leading to one-lock-many-keys interactions [34]. A recent study reviews different interaction mechanisms for unfolded and folded proteins [35]. Unfolded proteins are characterized by induced-fit interactions, whereas conformational change interactions characterize folded proteins. Although the recent literature does not explicitly separate IDPs from IDPRs, it is reasonable to hypothesize that IDPRs typically undergo conformational changes after post-translational modifications, whereas high-specificity and low-affinity induced fit mainly characterize IDPs.
Many studies aiming at a general classification of IDPs’ functional roles were based on the required presence of long disordered regions in the sequence [19–21,43–46]. However, the presence of a long disordered segment does not necessarily imply that a protein lacks a three-dimensional structure. In support of this claim, Gsponer et al. showed that also the percentage of disordered residues plays an important role in determining the half-life of a protein [47]. Therefore, both the length of disordered domains and the overall fraction of disordered residues in the sequence are critical parameters to distinguish different types of protein disorder.
Following clear-cut criteria inspired by experimental observations [15,47], we compiled two lists of human IDPs and IDPRs. We identified IDPs as those proteins having more than 30% of disordered residues (implying a shorter half-life [47]). We defined IDPRs as proteins with at least one long disordered segment (suggesting a higher sensitivity to proteolytic degradation [15]) but less than 30% of disordered residues in their sequence. We have assessed both the over-representation and the enrichment of IDPRs and IDPs in different functional classes and gene ontologies. Moreover, we have investigated the relative occurrence of IDPRs and IDPs in proteins related to cancer, cardiovascular, and neurodegenerative diseases.
Our results indicate that IDPRs and IDPs are structurally different, have different functional spectra, and play different roles in human diseases.
Materials and methods
Dataset of protein sequences
We downloaded the human proteome from the UniProt-SwissProt database (http://www.uniprot.org/uniprot) [48], release 2018_07. We selected 20386 human proteins by searching for reviewed proteins belonging to Homo Sapiens (Organism ID: 9606, Proteome ID: UP000005640).
Dataset of protein sequences associated to diseases
To select the proteins associated to cancer, cardiovascular, and neurodegenerative diseases, we used the lists of keywords found in Iakoucheva et al. [49], Cheng et al. [50], and Uversky [51]. Then, we extracted the proteins annotated with those keywords from the UniProt-SwissProt database. The lists of disease-related proteins are available in S2, S3 and S4 Texts.
Prediction of protein disorder
We identified disordered residues in the protein sequences using MobiDB (http://mobidb.bio.unipd.it) [52], a consensus database that combines experimental data (especially from X-ray crystallography, nuclear magnetic resonance (NMR) and cryo-electron microscopy (Cryo-EM)), curated data and disorder predictions based on various methods. From the initial dataset, we discarded proteins not annotated in MobiDB. Of the 20386 initial proteins, we analyzed 20030 proteins, i.e. 98.3% of the initial dataset.
Identification of IDPs and IDPRs
To partition the human proteome into different protein variants, we used two parameters: the percentage of disordered residues and the length of the longest disordered domain in the sequence. Based on these two parameters, we defined:
ordered proteins (ORDPs): they have less than 30% of disordered residues, no C- or N-terminal segments longer than 30 consecutive disordered residues as well as no segments longer than 40 consecutive disordered residues in positions distinct from the N- and C-terminus;
proteins with intrinsically disordered regions (IDPRs): they have less than 30% of disordered residues in the polypeptide chain and at least either one C- or N-terminal segment longer than 30 consecutive disordered residues or one segment longer than 40 consecutive disordered residues in positions distinct from the N- and C-terminus;
intrinsically disordered proteins (IDPs): they have more than 30% of disordered residues in the polypeptide chain.
Generally speaking, ORDPs are proteins with a limited number of disordered residues and the absence of disordered domains. IDPRs, unlike ORDPs, are proteins with at least one long disordered segment (implying a short half-life [15]) accommodated in globally folded structures. The rationale of taking into account the location of the long disordered segments in the definitions i) and ii) is discussed below in the discussion section. IDPs are proteins with a significant percentage of disorder (implying an unfolded structure and a high susceptibility to proteolytic degradation [47]).
Clustering of the variants of disorder through functional profiles
The functional profiles of ORDPs, IDPRs and IDPs were defined considering several annotations, such as: biological processes, cellular components and molecular functions from the Gene Ontology database [53] and protein classes from the PANTHER database [54–56].
Functional profiles associated with each protein variant are unitary vectors whose components are estimated as the number of proteins of a variant in each functional class divided by the number of proteins in that specific variant. Functional distances between protein variants were evaluated through the Euclidean distance of their profiles. The protein variants were then clustered using the average linkage hierarchical clustering algorithm [57]. The stability of the clustering against noise in the attribution of proteins to the different functional classes was assessed through bootstrap [58]. In each bootstrap re-sampling, new lists of ORDPs, IDPRs, and IDPs were compiled by randomly picking-up (with repetition) proteins in each variant. The robustness of two variants clustering together was estimated by f, the number of times this happened, divided by the number of bootstrap replicates [59]. Similarly, we evaluated the probability that two variants cluster just by chance by evaluating the fraction f of times that the two variants are clustered using resampled lists of ORDPs, IDPRs, and IDPs in which proteins from the entire human proteome are randomly inserted.
Over-representation of a protein variant in a functional class
The fractions of ORDPs, IDPRs, and IDPs were evaluated in each functional class. A protein variant is over-represented in a functional class if the frequency of its proteins in such a class is higher than the frequency of the same variant in the human proteome. The statistical significance of the differences was assessed through a binomial test [54,60]. We did three statistical analyses, one for each protein variant. A conservative p-value, accounting for the multiple testing, was obtained through Bonferroni correction [61].
Enrichment of a protein variant in a functional class
A functional class is enriched in the protein variant that has the highest frequency and depleted in the protein variant that has the lowest frequency. We evaluated the statistical significance of enrichment/depletion through a cascade of tests. Firstly, we tested the null-hypothesis that the three variants (ORDPs, IDPRs, and IDPs) have the same frequencies in a functional class. Through a goodness-of-fit (chi-square) test we rejected the null hypothesis when the p-value is lower than 0.05. Secondly, we tested the two pairs of variants with the highest and the two pairs with the lowest frequencies with two further goodness-of-fit tests of the hypothesis of equal probabilities. In each of the two tests, if the p-value is lower than 0.05 then the hypothesis of equal frequency of the pairs is discarded. Eventually we concluded that a given functional class is enriched in the protein variant that has the highest significant frequency and depleted in the protein variant that has the lowest significant frequency. Looking at S6 Table it is clear that, if the first test is not passed no conclusion on the enrichment can be made. Once the first test is passed then three cases are possible in each functional class: i) neither significant enrichment nor depletion; ii) either significant enrichment or significant depletion; iii) both significant enrichment and significant depletion.
Results
ORDPs, IDPRs and IDPs in the human proteome
In Table 1 we report numbers and frequencies of ORDPs, IDRPs, and IDPs in the human proteome. Most human proteins are ORDPs, followed by IDPs and IDPRs. The frequency of IDPs (proteins with more than 30% of disordered residues) is about 32%, a result consistent with Colak et al. [62]. According to previous estimates [63], the percentage of all proteins containing disorder (IDPRs and IDPs) is roughly 51%. Therefore, the classification of IDPRs either as ordered or disordered is important to correctly estimate the overall percentage of protein intrinsic disorder in the human proteome.
Table 1. Numbers and frequencies of ORDPs, IDRPs, and IDPs in the human proteome.
| Variant | Number of proteins | % |
|---|---|---|
| ORDPs | 9702 | 49 |
| IDRPs | 3862 | 19 |
| IDPs | 6466 | 32 |
| Total | 20030 | 100 |
Number and frequency of ORDPs, IDPRs and IDPs in the human proteome.
Protein variants: Distance of functional profiles
Functional distances between protein variants are reported in Fig 1 as hierarchical trees (distance matrices are reported in S1 Table) relative to gene ontology biological processes, cellular components, molecular functions, and to the PANTHER protein classes. IDPRs robustly cluster together with ORDPs. We conclude that, as a general trait, IDPRs have functional profiles which are more similar to those of ORDPs than to those of IDPs.
Fig 1. Clusters of the protein variants in the human proteome.
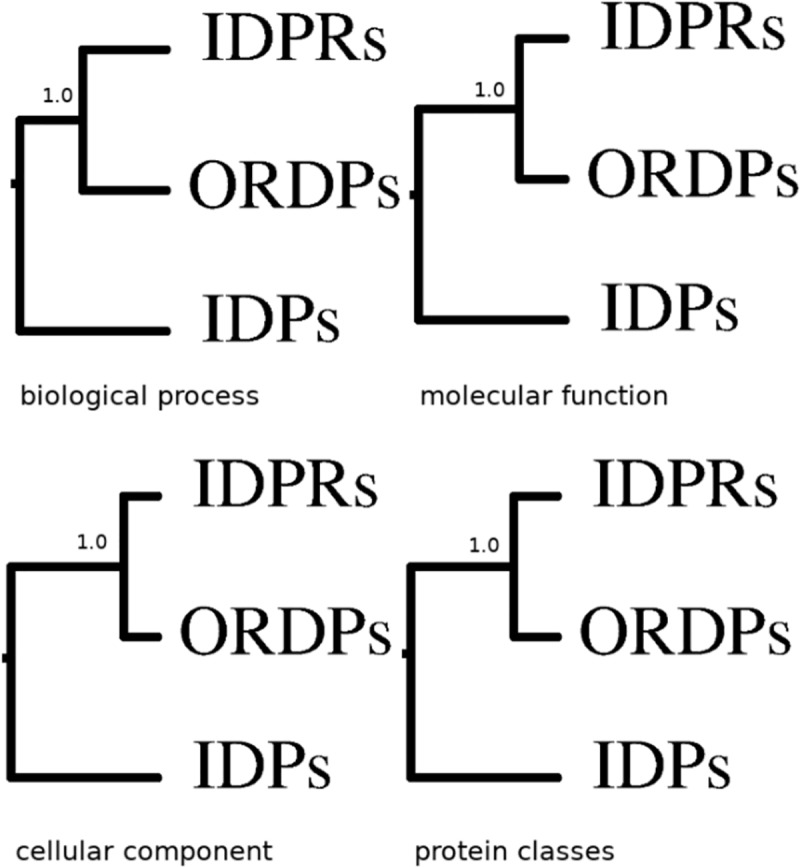
Hierarchical trees of ORDPs, IDPRs, and IDPs, based on different functional profiles (i.e. biological processes, molecular functions, cellular components, and PANTHER protein classes) are here reported. The length of the branches are not identical and reflect the distance matrices in S1 Table. In all the four cases, ORDPs and IDPRs are robustly separated, as indicated by bootstrap scores, the number of times these two groups clustered together divided by the number of bootstrap replicates (1000).
To further check that ORDPs and IDPRs do not cluster together by chance, we compared the hierarchical clusters of ORDPs, IDPRs, and IDPs in Fig 1 with the clusters obtained by repeatedly resampling each variant with proteins randomly selected from the human proteome. f values are comprised between 0.25 and 0.37 (see Figure A in S1 Text). We, therefore, concluded that the lower distance between the functional profiles of ORDPs and IDPRs, observed in the human proteome (Fig 1), is due to functional specialization and not by chance.
Protein variants: Over-representation in the functional classes
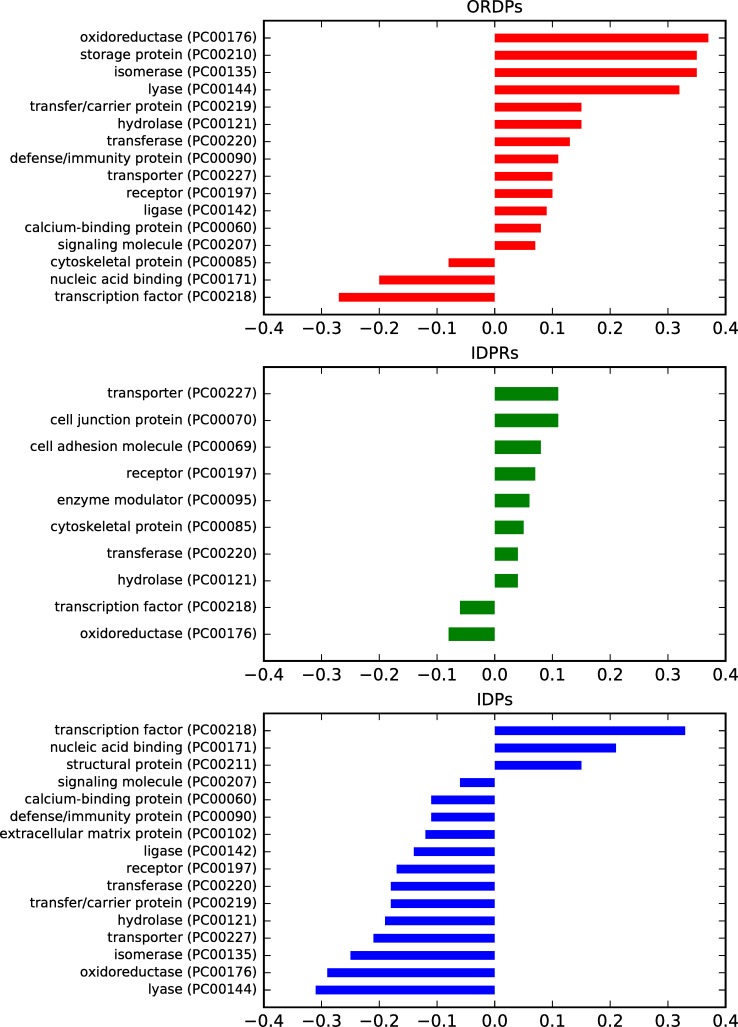
A protein variant is over-represented (under-represented) in a functional class if its frequency is significantly higher (lower) than the frequency it has in the human proteome. In Fig 2 and S2 Table, we report the over- (under-) representation of ORDPs, IDPRs, and IDPs in the functional classes of PANTHER.
Fig 2. Over- (under-) representation of the protein variants in the PANTHER protein classes.
Bar charts of the normalized differential occurrence of ORDPs, IDPRs, and IDPs in various protein classes, with respect to the human proteome (the reference). Only statistically significant differences are reported (p-value <0.05).
As expected, ORDPs are over-represented among enzymes (oxidoreductases, isomerases, lyases, hydrolases, transferases, ligases), and in several other protein classes related to storage, defense/immunity, transporters, receptors, transfer/carriers and calcium-binding proteins. ORDPs are under-represented among cytoskeletal proteins, nucleic acid binding proteins and transcription factors.
IDPRs are over-represented among transporters, cell-junction, cell adhesion, receptors, enzyme modulators, cytoskeletal proteins. Moreover, they are over-represented in enzymes such as hydrolases and transferases. IDPRs are under-represented among transcription factors and oxidoreductases. Notably, both ORDPs and IDPRs are over-represented in transporters, receptors, hydrolases, and transferases; interestingly, IDPs are under-represented in all these latter functional classes.
IDPs are over-represented only among structural proteins, transcription factors, and nucleic-acid binding proteins; note that the two latter functions are not over-represented both in ORDPs and IDPRs.
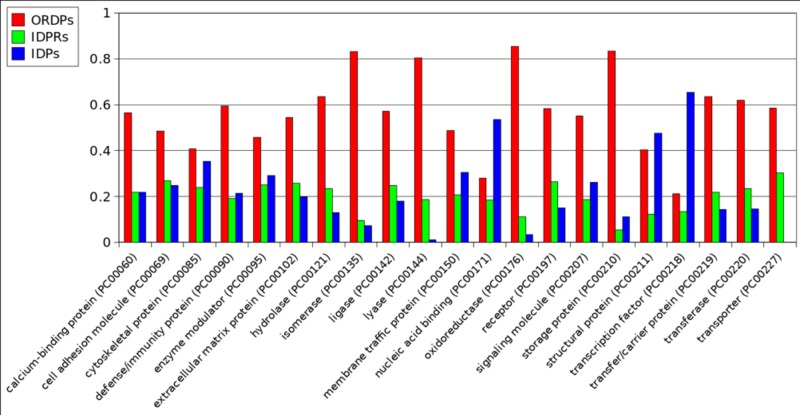
We also compared the over- (under-) representation of the variants in Gene Ontology annotations related to biological processes, molecular functions and cellular components (for details see: S1, S2 and S3 Figs; S3, S4 and S5 Tables).
In a nutshell, it is worth noting that each variant displays articulated spectra of specific GO annotations, that are generally consistent with the closeness of IDPRs to ORDPs already shown above, based on functional distances and PANTHER classes. On the contrary, IDPs have a well characterized, peculiar, set of annotations. Indeed, IDPs are specifically over-represented in molecular functions related to nucleic acid binding and chromatin binding, and developmental biological processes (i.e., pattern specification processes, ectoderm, embryo, mesoderm and system development), where ORDPs are under-represented and IDPRs are not significantly represented.
Enrichment of functional classes in protein variants
In each protein functional class, the three protein variants (ORDPs, IDPRs, IDPs) are differently distributed (Fig 3). A functional class is enriched (depleted) in the variant that has the highest (lowest) relative frequency. The statistical significance of the enrichment (depletion) is evaluated with a specific test (see Materials and Methods). In Fig 3 and S6 Table, we report the protein classes that reached statistical significance in the assessment of their enrichment (depletion). Only two protein classes are enriched in IDPs: nucleic acid binding proteins and transcription factors. The other classes are enriched in ORDPs. No protein class is enriched in IDPRs.
Fig 3. Functional classes are differently enriched in ORDPs, IDPRs, and IDPs.
A functional class is enriched (depleted) in the protein variant that has significantly the highest (lowest) frequency. All the classes shown in this figure passed the enrichment (depletion) test for at least one variant (detailed numerical values can be found in S6 Table).
The results on the enrichment of biological processes, cellular components, and molecular functions in the protein variants are reported in S7, S8 and S9 Tables, respectively. Many development processes (pattern specification, ectoderm, mesoderm, endoderm, and system development), chromatin and nucleic acid binding are enriched in IDPs whereas ORDPs enrich anatomical structure morphogenesis, death, and cell differentiation. Notably, IDPRs enrich only calcium-dependent phospholipid binding and helicase activity. Finally, IDPs and IDPRs do not enrich any cellular component.
Over-representation and enrichment of protein variants in diseases
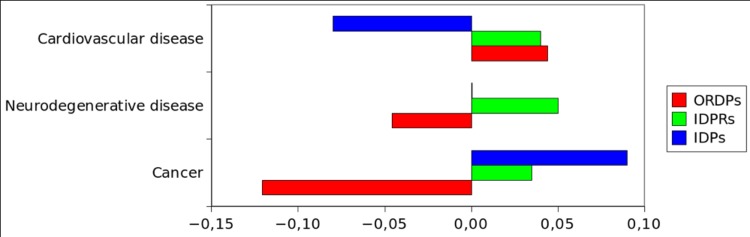
In Fig 4 and S10 Table, we report the over-representation of the protein variants (ORDPs, IDPRs, and IDPs) among the proteins related to cardiovascular diseases, neurodegenerative diseases, and cancer. IDPs are over-represented in cancer and under-represented in cardiovascular diseases. On the contrary, ORDPs are over-represented in cardiovascular diseases and under-represented in cancer. IDPRs are over-represented in all the diseases here considered, and particularly in neurodegenerative diseases.
Fig 4. Over-representation of the variants of disorder in diseases.
Bar charts of the normalized differential occurrence (with respect to the human proteome) of ORDPs, IDPRs, and IDPs in three groups of disease-related proteins.
In Fig 5 and S11 Table, we report the enrichment of protein variants in diseases. Notably, cancer-related proteins are specifically enriched in IDPs. Both, proteins related to cardiovascular diseases and those related to neurodegenerative diseases are enriched in ORDPs. IDPRs are depleted in all three categories of disease-related proteins (not significantly among proteins related to cardiovascular diseases, see S11 Table).
Fig 5. Enrichment of the protein variants in diseases.
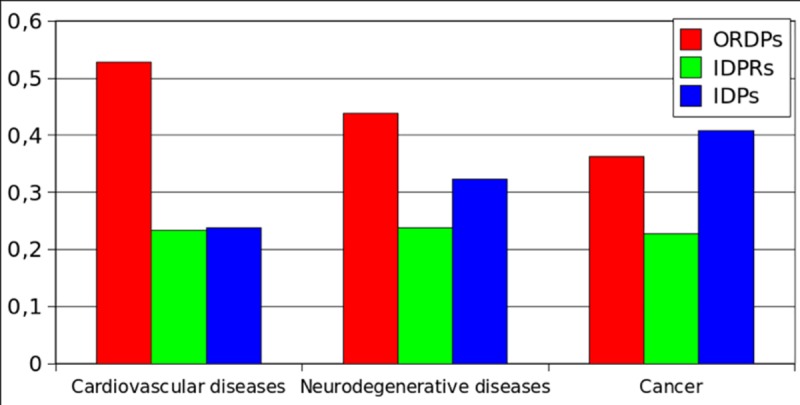
Different groups of disease-related proteins are enriched (depleted) in the protein variant that has significantly the highest (lowest) frequency. Each group is significantly enriched (depleted) in at least one of the variants (detailed numerical values can be found in S11 Table).
Discussion
In this study we operationally distinguish the functional spectra and biological roles of three broad variants of intrinsic protein disorder: i) ordered proteins (ORDPs), ii) structured proteins with intrinsically disordered protein regions (IDPRs), and iii) intrinsically disordered proteins (IDPs). The focus of this work is on the functional distinction and the different roles in diseases of IDPs and IDPRs, as we distinguish them.
Many studies aiming at a general classification of functional roles of protein intrinsic disorder were based on the required presence of long disordered regions in the sequence, thus considering IDPRs and IDPs as a single entity [19–21,43–46].
However, the presence of just one long disordered segment (longer than 30 disordered residues) does not imply that a protein is globally intrinsically disordered, i.e., that it lacks a stable three-dimensional structure. With this criterion, it is impossible to discriminate between IDPs and IDPRs. Moreover, the general requirement of 30 consecutive disordered residues as a fingerprint of IDPs is neither experimentally justified nor universally accepted in the literature. Indeed, this length ranges from 20 residues in Necci et al. [64] up to 50 residues, as in Dunker et al. [3] and Ward et al. [44]. Our distinction is based precisely on this point: the difference between IDPs and IDPRs depends on the relative proportion of the unstructured regions. It has been shown that proteins with more than 30% of disordered residues are more prone to proteolytic degradation [47], likely due to the absence of a well-defined tertiary structure. In line with this argument and in agreement with other studies that adopt the same threshold [62,65], we have then defined proteins with more than 30% of disordered residues as IDPs. Finally, to identify IDPRs, we considered the study by van Der Lee et al. [15]. They report that a protein has a shorter half-life (due to the protease-vulnerability of its disordered regions) either if it has a segment of 30 consecutive disordered residues located at the C- or N-terminus or a segment of 40 consecutive disordered residues in a generic position of the polypeptide chain. Moreover, unstructured regions longer than ~25 residues have been considered to evolve, function, and exist as independent, disordered domains [16,66,67]. Thus, we identified IDPRs as those proteins with less than 30% of disordered residues but with at least one disordered domain.
The main result of this study is that intrinsically disordered proteins (IDPs) and structured proteins with intrinsically disordered protein regions (IDPRs) have different functional roles in the cell. We found that IDPs are mainly associated with nucleic acid binding proteins, chromatin binding proteins, transcription factors, and many developmental processes. The functional classes in which IDPRs are over-represented substantially overlap with those in which ORDPs are also over-represented (transporters, receptors, and catalytic activities). This outcome shows that the functional roles of IDPRs are more similar to those of ORDPs than to those of IDPs. Moreover, IDPs are specifically over-represented in PANTHER functional classes and GO annotations where both IDPRs and ORDPs are under-represented. We conclude that the broadly shared statement that IDPs are specifically involved in signaling and cell-regulation [68] should be taken with caution.
A few remarks are in order at this point of the discussion. We have reminded in the introduction that, as a general trait, IDPs do not contain sufficient hydrophobic amino acids [2], which are needed to mediate co-operative folding of polypeptide chains into a well-defined tertiary structure. Moreover, intrinsic disorder can have different levels of protein structural organization, and whole proteins, or various protein regions can be disordered to a different degree [69]. To reconstruct a perspective, it is also worth reminding that since the time in which the first DNA and protein structures were determined, in the early fifties of the past century, molecular biology and biophysics have been dominated by the static picture brought by the crystallographers. Notwithstanding the importance of static equilibrium structures, the view that there are functional motions in proteins, spanning many scales of time, from femtoseconds to seconds, gradually emerged [70–74]. These works were influential in suggesting a thermodynamic scenario for proteins, in which fluctuations had a relevant role. In particular G. Careri [75] had the intuition that enzyme catalysis could be understood within a thermodynamic setting based on fluctuation-dissipation theorems and on a theory of correlated fluctuations.
The nowadays prevailing picture is that proteins acquire functions within a conformational continuum [15]. Thus, IDPs and IDPRs can be related to dynamic structures that interconvert on multiple timescales, covering a spectrum of states that are in dynamic equilibrium under physiological conditions [76].
ORDPs, IDPRs and IDPs are here coarse-grained operational classifications into which to accommodate different broad functional classes, that are influenced by the degree of disorder [77]. Now, it is quite clear that a polypeptide chain can encode for highly heterogeneous (in space and time) protein molecules containing unique sets of foldons (i.e. ordered segments), semi-foldons (i.e., partially folded protein segments with transient residual structure), inducible foldons (i.e., segments that are able to fold at interaction with specific binding partners), nonfoldons (i.e., protein segments with functions depending on their intrinsically disordered nature), and unfoldons (i.e., segments that are able to unfold under specific conditions, becoming functional) [69,77]. Of this mosaic of units we do not have yet a unifying (equilibrium/nonequilibrium) thermodynamic theory, based on basic statistical models. Finding these models, possibly in a mesoscopic stochastic theory of heterogeneous quasi-random polymers (see e.g. [78]), could provide an understanding of the operational rules we used to define ORDs, IDPRs and IDPs.
As regards to disease-related proteins, we observe that IDPs are abundant among cancer-related proteins but not among proteins associated with cardiovascular and neurodegenerative diseases, where ORDPs seem to dominate. This observation better specifies the quite established D2 concept [33].
Overall, our approach is useful to discriminate between IDPs and IDPRs operationally, and we have used it to demonstrate the different functional roles of these two variants in the human proteome. As a conclusion, we believe that it is important to correctly evaluate the occurrence of disorder in proteomes, in evolutionary studies, in understanding functional aspects related to different flavors of protein disorder, and in deciphering the role of these variants in the emergence of diseases.
Supporting information
Bar charts of the normalized differential occurrence of ORDPs, IDPRs, and IDPs in various biological processes, with respect to the human proteome (the reference). Only statistically significant differences are reported (p-value <0.05).
(TIF)
Bar charts of the normalized differential occurrence of ORDPs, IDPRs, and IDPs in various molecular functions, with respect to the human proteome (the reference). Only statistically significant differences are reported (p-value <0.05).
(TIF)
Bar charts of the normalized differential occurrence of ORDPs, IDPRs, and IDPs in various cellular components, with respect to the human proteome (the reference). Only statistically significant differences are reported (p-value <0.05).
(TIF)
(DOCX)
(DOCX)
The asterix near some biological process indicates that the over-representation test is not statistically significant.
(DOCX)
The asterix near some molecular function indicates that the over-representation test is not statistically significant.
(DOCX)
(DOCX)
The protein classes for which the enrichment test is not statistically significant are reported in red.
(DOCX)
The biological processes for which the enrichment test is not statistically significant are reported in red.
(DOCX)
The molecular functions for which the enrichment test is not statistically significant are reported in red.
(DOCX)
The cellular components for which the enrichment test is not statistically significant are reported in red.
(DOCX)
The diseases for which the over-representation test is not statistically significant are reported in red.
(DOCX)
The diseases for which the enrichment test is not statistically significant are reported in red.
(DOCX)
(DOCX)
Lists of the cancer-related proteins found in Iakoucheva et al. [49] and considered in this work.
(TXT)
Lists of the cardiovascular-related proteins found in Cheng et al. [50] and considered in this work.
(TXT)
Lists of the neurodegenerative-related proteins found in Uversky [51] and considered in this work.
(TXT)
Abbreviations
- ORDPs
ordered proteins
- IDPRs
structured proteins with intrinsically disordered regions
- IDPs
intrinsically disordered proteins
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
Publication of this study was funded in part by a basic research grant to AG from the Sapienza University of Rome (000008_17_RS_PICCOLI_2016_GIANSANTI-RICERCA 2016). There were no additional sources of funding. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wright PE, Dyson HJ. Intrinsically unstructured proteins: re-assessing the protein structure-function paradigm. J Mol Biol. 1999;31:293–321. [DOI] [PubMed] [Google Scholar]
- 2.Uversky VN, Gillespie JR, Fink AL. Why Are “Natively Unfolded” Proteins Unstructured Under Physiologic Conditions? PROTEINS: Structure, Function, and Genetics 2000;41:415–427. [DOI] [PubMed] [Google Scholar]
- 3.Dunker AK, Lawson JD, Brown CJ, Williams RM, Romero P, Oh JS, et al. Intrinsically disordered protein. J Mol Graph Model. 2001;19:26–59. [DOI] [PubMed] [Google Scholar]
- 4.Tompa P. Intrinsically unstructured proteins. Trends Biochem Sci. 2002;27:527–33. [DOI] [PubMed] [Google Scholar]
- 5.Fink AL. Natively unfolded proteins. Curr Opin Struct Biol. 2005;15:35–41. 10.1016/j.sbi.2005.01.002 [DOI] [PubMed] [Google Scholar]
- 6.Daughdrill GW, Pielak GJ, Uversky VN, Cortese MS, Dunker KA. Natively Disordered Proteins In: Protein Folding Handbook. Wiley-Blackwell; 2008. [Google Scholar]
- 7.Tompa P, Fersht A. Structure and function of intrinsically disordered proteins. New York: Chapman and Hall/CRC; 2009. [Google Scholar]
- 8.Schlessinger A, Schaefer C, Vicedo E, Schmidberger M, Punta M, Rost B. Protein disorder—a breakthrough invention of evolution?. Curr Opin Struct Biol. 2011;21:412–418. 10.1016/j.sbi.2011.03.014 [DOI] [PubMed] [Google Scholar]
- 9.Babu MM. The contribution of intrinsically disordered regions to protein function, cellular complexity, and human disease. Biochemical Society Transactions. 2016;44:1185–1200. 10.1042/BST20160172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uversky VN. Intrinsic Disorder, Protein–Protein Interactions, and Disease. Advances in Protein Chemistry and Structural Biology. 2017;1876–1623. [DOI] [PubMed] [Google Scholar]
- 11.Uversky VN. Paradoxes and wonders of intrinsic disorder: Stability of instability. Intrinsically Disordered Proteins. 2017; 5(1):e1327757 10.1080/21690707.2017.1327757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Gall T, Romero PR, Cortese MS, Uversky VN, Dunker AK. Intrinsic disorder in the Protein Data Bank. J Biomol Struct Dyn. 2007;24(4):325–42. 10.1080/07391102.2007.10507123 [DOI] [PubMed] [Google Scholar]
- 13.DeForte S, Uversky VN. Resolving the ambiguity: Making sense of intrinsic disorder when PDB structures disagree. Protein Sci. 2015;25(3):676–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uversky VN, Dunker AK. Understanding protein non-folding. Biochim Biophys Acta. 2010;1804:1231–64. 10.1016/j.bbapap.2010.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van der Lee R, Lang B, Kruse K, Gsponer J, Sanchez de Groot N, Huynen MA, et al. Intrinsically disordered segments affect protein half-life in the cell and during evolution. Cell Rep. 2014;8:1832–44. 10.1016/j.celrep.2014.07.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oldfield CJ, Dunker AK. Intrinsically disordered proteins and intrinsically disordered protein regions. Annu Rev Biochem. 2014;83:553–84. 10.1146/annurev-biochem-072711-164947 [DOI] [PubMed] [Google Scholar]
- 17.Uversky VN. Intrinsic disorder here, there, and everywhere, and nowhere to escape from it. Cell Mol Life Sci. 2017;74:3065–3067. 10.1007/s00018-017-2554-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunker K, Babu MM, Barbar E, Blackledge M, Bondos SE, Dosztányi Z et al. What’s in a name? Why these proteins are intrinsically disordered. Intrinsically Disordered Proteins. 2013:1:e24157 10.4161/idp.24157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie H, Vucetic S, Iakoucheva LM, Oldfield CJ, Dunker AK, Uversky VN, et al. Functional anthology of intrinsic disorder. 1. Biological processes and functions of proteins with long disordered regions. J Proteome Res. 2007;6:1882–98. 10.1021/pr060392u [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vucetic S, Xie H, Iakoucheva LM, Oldfield CJ, Dunker AK, Obradovic Z, et al. Functional anthology of intrinsic disorder. 2. Cellular components, domains, technical terms, developmental processes, and coding sequence diversities correlated with long disordered regions. J Proteome Res. 2007;6:1899–916. 10.1021/pr060393m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie H, Vucetic S, Iakoucheva LM, Oldfield CJ, Dunker AK, Obradovic Z, et al. Functional anthology of intrinsic disorder. 3. Ligands, post-translational modifications, and diseases associated with intrinsically disordered proteins. J Proteome Res. 2007;6:1917–32. 10.1021/pr060394e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunker AK, Oldfield CJ, Meng J, Romero P, Yang JY, Chen JW, et al. The unfoldomics decade: an update on intrinsically disordered proteins. BMC Genomics. 2008;9 Suppl 2:S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan J, Mizianty MJ, Filipow PL, Uversky VN, Kurgan L. RAPID: fast and accurate sequence-based prediction of intrinsic disorder content on proteomic scale. Biochim Biophys Acta. 2013;1834:1671–80. 10.1016/j.bbapap.2013.05.022 [DOI] [PubMed] [Google Scholar]
- 24.Uversky VN, Oldfield CJ, Dunker AK. Showing your ID: intrinsic disorder as an ID for recognition, regulation and cell signaling. J Mol Recognit. 2005;18:343–84. 10.1002/jmr.747 [DOI] [PubMed] [Google Scholar]
- 25.Garza AS, Ahmad N, Kumar R. Role of intrinsically disordered protein regions/domains in transcriptional regulation. Life Sci. 2009;84:189–93. 10.1016/j.lfs.2008.12.002 [DOI] [PubMed] [Google Scholar]
- 26.Uversky VN. The mysterious unfoldome: structureless, underappreciated, yet vital part of any given proteome. J Biomed Biotechnol. 2010;2010:568068 10.1155/2010/568068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dyson HJ, Wright PE. Intrinsically Unstructured Proteins and Their Functions. Nat Rev. 2005;6:197–208. [DOI] [PubMed] [Google Scholar]
- 28.Buljan M, Chalancon G, Dunker AK, Bateman A, Balaji S, Fuxreiter M, et al. Alternative splicing of intrinsically disordered regions and rewiring of protein interactions. Curr Opin Struct Biol. 2013;23:443–50. 10.1016/j.sbi.2013.03.006 [DOI] [PubMed] [Google Scholar]
- 29.Hegyi H, Kalmar L, Horvath T, Tompa P. Verification of alternative splicing variants based on domain integrity, truncation length and intrinsic protein disorder. Nucleic Acids Res. 2011;39:1208–19. 10.1093/nar/gkq843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou J, Zhao S, Dunker AK. Intrinsically Disordered Proteins Link Alternative Splicing and Post-translational Modifications to Complex Cell Signaling and Regulation. J Mol Biol. 2018;430:2342–59. 10.1016/j.jmb.2018.03.028 [DOI] [PubMed] [Google Scholar]
- 31.Uversky VN, Oldfield CJ, Midic U, Xie H, Xue B, Vucetic S, et al. Unfoldomics of human diseases: linking protein intrinsic disorder with diseases. BMC Genomics. 2009;10 Suppl 1:S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uversky VN, Dave V, Iakoucheva LM, Malaney P, Metallo SJ, Pathak RR, et al. Pathological unfoldomics of uncontrolled chaos: intrinsically disordered proteins and human diseases. Chem Rev. 2014;114:6844–79. 10.1021/cr400713r [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uversky VN, Oldfield CJ, Dunker AK. Intrinsically disordered proteins in human diseases: introducing the D2 concept. Annu Rev Biophys. 2008;37:215–46. 10.1146/annurev.biophys.37.032807.125924 [DOI] [PubMed] [Google Scholar]
- 34.Darling AL, Uversky VN. Intrinsic Disorder and Posttranslational Modifications: The Darker Side of the Biological Dark Matter. Front Genet. 2018;9:158 10.3389/fgene.2018.00158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arai M. Unified understanding of folding and binding mechanisms of globular and intrinsically disordered proteins. Biophys Rev. 2018;10:163–81. 10.1007/s12551-017-0346-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fisher E. Einfluss der Configuration auf die Wirkung der Enzyme. Berichte der deutschen chemischen Gesellschaft. 1894,27:2985–93. [Google Scholar]
- 37.Borgia A, Borgia MB, Bugge K, Kissling VM, Heidarsson PO, Fernandes CB et al. Extreme disorder in an ultra-high-affinity protein complex. Nature. 2018;555(7694):61–66. 10.1038/nature25762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uversky VN. Natively unfolded proteins: a point where biology waits for physics. Protein Sci. 2002;11:739–56. 10.1110/ps.4210102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kulkarni PK, Uversky VN. Intrinsically Disordered Proteins: The Dark Horse of the Dark Proteome. Proteomics. 2018;18:1800061. [DOI] [PubMed] [Google Scholar]
- 40.Gao J, Xu D. Correlation between posttranslational modification and intrinsic disorder in protein. Pac Symp Biocomput. 2012;94–103. [PMC free article] [PubMed] [Google Scholar]
- 41.Pejaver V, Hsu W-L, Xin F, Dunker AK, Uversky VN, Radivojac P. The structural and functional signatures of proteins that undergo multiple events of post-translational modification. Protein Sci. 2014;23:1077–93. 10.1002/pro.2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burgi J, Xue B, Uversky VN, van der Goot FG. Intrinsic Disorder in Transmembrane Proteins: Roles in Signaling and Topology Prediction. PLoS One. 2016;11:e0158594 10.1371/journal.pone.0158594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tompa P, Fersht A. Structure and function of intrinsically disordered proteins. CRC Press, Taylor & Francis Group; 2010. [Google Scholar]
- 44.Ward JJ, McGuffin LJ, Bryson K, Buxton BF, Jones DT. The DISOPRED server for the prediction of protein disorder. Bioinformatics. 2004;20:2138–2139. 10.1093/bioinformatics/bth195 [DOI] [PubMed] [Google Scholar]
- 45.Ward JJ, Sodhi JS, McGuffin LJ, Buxton BF, Jones DT. Prediction and functional analysis of native disorder in proteins from the three kingdoms of life. J Mol Biol. 2004;337:635–45. 10.1016/j.jmb.2004.02.002 [DOI] [PubMed] [Google Scholar]
- 46.Peng Z, Yan J, Fan X, Mizianty MJ, Xue B, Wang K, Hu G, Uversky VN, Kurgan L. Exceptionally abundant exceptions: comprehensive characterization of intrinsic disorder in all domains of life. Cell Mol Life Sci. 2015;72(1):137–51. 10.1007/s00018-014-1661-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gsponer J, Futschik ME, Teichmann SA, Babu MM. Tight regulation of unstructured proteins: from transcript synthesis to protein degradation. Science. 2008;322:1365–8. 10.1126/science.1163581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.The UniProt Consortium. UniProt: the universal protein knowledgebase. Nucleic Acids Res. 2017;45:D158–69. 10.1093/nar/gkw1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iakoucheva LM, Brown CJ, Lawson JD, Obradovic Z, Dunker AK. Intrinsic disorder in cell-signaling and cancer-associated proteins. J Mol Biol. 2002;323:573–84. 10.1016/s0022-2836(02)00969-5 [DOI] [PubMed] [Google Scholar]
- 50.Cheng Y, LeGall T, Oldfield CJ, Dunker AK, Uversky VN. Abundance of intrinsic disorder in protein associated with cardiovascular disease. Biochemistry. 2006;45:10448–60. 10.1021/bi060981d [DOI] [PubMed] [Google Scholar]
- 51.Uversky VN. The triple power of D(3): protein intrinsic disorder in degenerative diseases. Front Biosci (Landmark Ed). 2014;19:181–258. [DOI] [PubMed] [Google Scholar]
- 52.Piovesan D, Tabaro F, Paladin L, Necci M, Micetic I, Camilloni C, et al. MobiDB 3.0: more annotations for intrinsic disorder, conformational diversity and interactions in proteins. Nucleic Acids Res. 2018;46:D471–6. 10.1093/nar/gkx1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–9. 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mi H, Muruganujan A, Casagrande JT, Thomas PD. Large-scale gene function analysis with the PANTHER classification system. Nat Protoc. 2013;8:1551–66. 10.1038/nprot.2013.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mi H, Poudel S, Muruganujan A, Casagrande JT, Thomas PD. PANTHER version 10: expanded protein families and functions, and analysis tools. Nucleic Acids Res. 2016;44:D336–342. 10.1093/nar/gkv1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mi H, Huang X, Muruganujan A, Tang H, Mills C, Kang D, et al. PANTHER version 11: expanded annotation data from Gene Ontology and Reactome pathways, and data analysis tool enhancements. Nucleic Acids Res. 2017;45:D183–9. 10.1093/nar/gkw1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Webb AR. Statistical Pattern Recognition, 2nd Edition John Wiley & Sons; 2002. [Google Scholar]
- 58.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Boca Raton, Florida, USA: Chapman & Hall/CRC; 1993. [Google Scholar]
- 59.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–91. 10.1111/j.1558-5646.1985.tb00420.x [DOI] [PubMed] [Google Scholar]
- 60.Armitage P. Statistical methods in medical research. Blackwell Scientific Oxford; 1971. [Google Scholar]
- 61.Bland M. An introduction to medical statistics. Oxford University Press Oxford; 1987. [Google Scholar]
- 62.Colak R, Kim T, Michaut M, Sun M, Irimia M, Bellay J, et al. Distinct types of disorder in the human proteome: functional implications for alternative splicing. PLoS Comput Biol. 2013;9:e1003030 10.1371/journal.pcbi.1003030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Niklas KJ, Dunker AK, and Yruela I. The evolutionary origins of cell type diversification and the role of intrinsically disordered proteins. Journal of Experimental Botany, 2018; 69,1437–1446. 10.1093/jxb/erx493 [DOI] [PubMed] [Google Scholar]
- 64.Necci M, Piovesan D, Tosatto SCE. Large-scale analysis of intrinsic disorder flavors and associated functions in the protein sequence universe. Protein Sci. 2016;25:2164–74. 10.1002/pro.3041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bellay J, Han S, Michaut M, Kim T, Costanzo M, Andrews BJ, et al. Bringing order to protein disorder through comparative genomics and genetic interactions. Genome Biol. 2011;12:R14 10.1186/gb-2011-12-2-r14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen JW, Romero P, Uversky VN, Dunker AK. Conservation of intrinsic disorder in protein domains and families: I. A database of conserved predicted disordered regions. J Proteome Res. 2006;5,879–887. 10.1021/pr060048x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tompa P, Fuxreiter M, Oldfield CJ, Simon I, Dunker AK, and Uversky VN. Close encounters of the third kind: disordered domains and the interactions of proteins. Bioessays. 2009;31,328–3. 10.1002/bies.200800151 [DOI] [PubMed] [Google Scholar]
- 68.Wright PE, Dyson HJ. Intrinsically Disordered Proteins in Cellular Signaling and Regulation. Nat Rev Mol Cell Biol. 2015;16:18–29. 10.1038/nrm3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Uversky VN. Under-Folded Proteins: conformational Ensembles and Their Roles in Protein Folding, Function, and Pathogenesis. Biopolymers 2013;99(11):870–887. 10.1002/bip.22298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weber G. Energetics of ligand binding to proteins. Adv. Prot. Chem. 1975:29:1–83 (1975). [DOI] [PubMed] [Google Scholar]
- 71.Careri G, Gratton E, Fasella P. Enzyme Dynamics: The Statistical Physics Approach. Ann. Rev. Biophysics Bioeng. 8, 69–97 (1979). [DOI] [PubMed] [Google Scholar]
- 72.Frauenfelder H, Petsko G, Tsernoglou D. Temperature-dependent X-ray diffraction as a probe of protein structural dynamics. Nature 1979;280;5558–5563. [DOI] [PubMed] [Google Scholar]
- 73.Brooks CI, Karplus M, Petitt B, Proteins: A Theoretical Perspective of Dynamics, Structure and Thermodynamics, Wiley, New York, 1988. [Google Scholar]
- 74.Kay LE. Protein dynamics from NMR. Nature. Struc. Biol. NMR Suppl. 1998;S5:513–517. [DOI] [PubMed] [Google Scholar]
- 75.Careri G. “The Fluctuating Enzyme” in Quantum Statistical Mechanics in the Natural Sciences, Springer; US. New York; 1974, pp. 15–35. [Google Scholar]
- 76.Daughdrill GW, Narayanaswami P, Gilmore SH, Belczyk A, Brown CJ. Dynamic behavior of an intrinsically unstructured linker domain is conserved in the face of negligible amino acid sequence conservation. J Mol Evol. 2007;65(3):277–88. 10.1007/s00239-007-9011-2 [DOI] [PubMed] [Google Scholar]
- 77.Uversky VN. Functional roles of transiently and intrinsically disordered regions within proteins. FEBS J. 2015;282(7):1182–9. 10.1111/febs.13202 [DOI] [PubMed] [Google Scholar]
- 78.Zhang X-J, Qian H, Qian M. Stochastic theory of nonequilibrium steady states and its applications. Part I. Phys. Rep. 2012;510:1–86. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bar charts of the normalized differential occurrence of ORDPs, IDPRs, and IDPs in various biological processes, with respect to the human proteome (the reference). Only statistically significant differences are reported (p-value <0.05).
(TIF)
Bar charts of the normalized differential occurrence of ORDPs, IDPRs, and IDPs in various molecular functions, with respect to the human proteome (the reference). Only statistically significant differences are reported (p-value <0.05).
(TIF)
Bar charts of the normalized differential occurrence of ORDPs, IDPRs, and IDPs in various cellular components, with respect to the human proteome (the reference). Only statistically significant differences are reported (p-value <0.05).
(TIF)
(DOCX)
(DOCX)
The asterix near some biological process indicates that the over-representation test is not statistically significant.
(DOCX)
The asterix near some molecular function indicates that the over-representation test is not statistically significant.
(DOCX)
(DOCX)
The protein classes for which the enrichment test is not statistically significant are reported in red.
(DOCX)
The biological processes for which the enrichment test is not statistically significant are reported in red.
(DOCX)
The molecular functions for which the enrichment test is not statistically significant are reported in red.
(DOCX)
The cellular components for which the enrichment test is not statistically significant are reported in red.
(DOCX)
The diseases for which the over-representation test is not statistically significant are reported in red.
(DOCX)
The diseases for which the enrichment test is not statistically significant are reported in red.
(DOCX)
(DOCX)
Lists of the cancer-related proteins found in Iakoucheva et al. [49] and considered in this work.
(TXT)
Lists of the cardiovascular-related proteins found in Cheng et al. [50] and considered in this work.
(TXT)
Lists of the neurodegenerative-related proteins found in Uversky [51] and considered in this work.
(TXT)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.