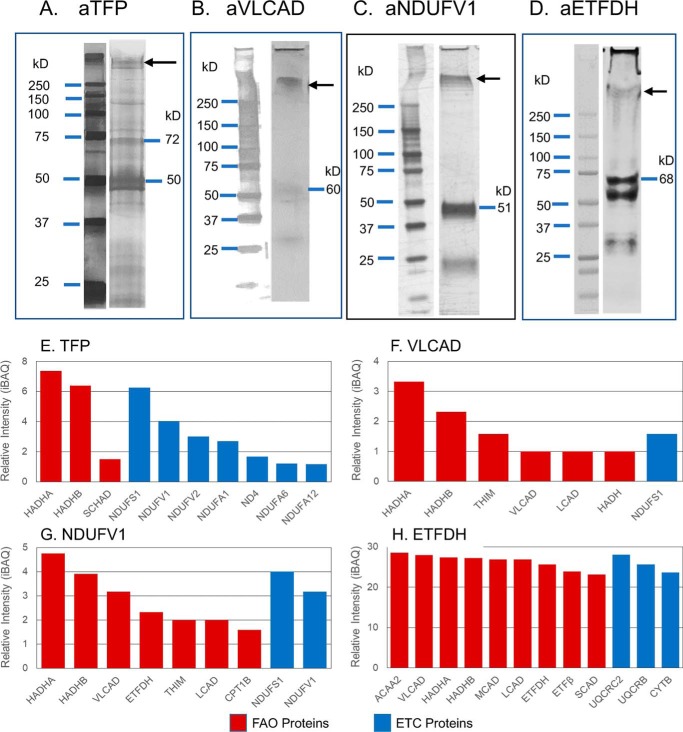
Figure 4.
Mass spectrometry analysis of proteins isolated by co-immunoprecipitation from mouse heart mitochondria treated with protein cross-linking agents. Rat heart mitochondria were incubated with the chemical cross-linker EGS, incubated with an anti-TFP, anti-VLCAD, anti-ETFDH, or anti-ETFDH antibody. The cross-linked products were co-immunoprecipitated using protein A–Sepharose 4B, followed by SDS-PAGE separation, in-gel protein digest, and MS analysis. A–D, Western blottings of the SDS-polyacrylamide gels with the indicated antisera identified larger molecular mass bands consistent with other proteins cross-linked to the primary antigen as seen to the right of A–D. The region of the SDS-polyacrylamide gel corresponding to that indicated by the arrows was cut from the gel and subjected to MS analysis. E–H, proteins identified by MS analysis of high-molecular mass bands from SDS-PAGE of cross-linked samples. Red bars represent FAO proteins, and blue bars represent ETC proteins. E, co-immunoprecipitation with anti-TFP antibody identified three complex I NADH-binding domain subunits. F, treatment with anti-VLCAD antibody also precipitated HADHA and HADHB subunits and ETC complex I subunit, NDUFS1, one of the NADH-binding domain subunits seen to bind to TFP. G, precipitation with an anti-NDUFV1 antibody precipitated HADHA and HADHB and NDUFS1 subunit of complex I. H, treatment with anti-ETFDH antibody precipitated one complex III subunit, core II, plus numerous membrane and matrix-associated FAO proteins (HADHA, HADHB, and VLCAD), along with other acyl-CoA dehydrogenases and ETF subunits.