Figure 4.
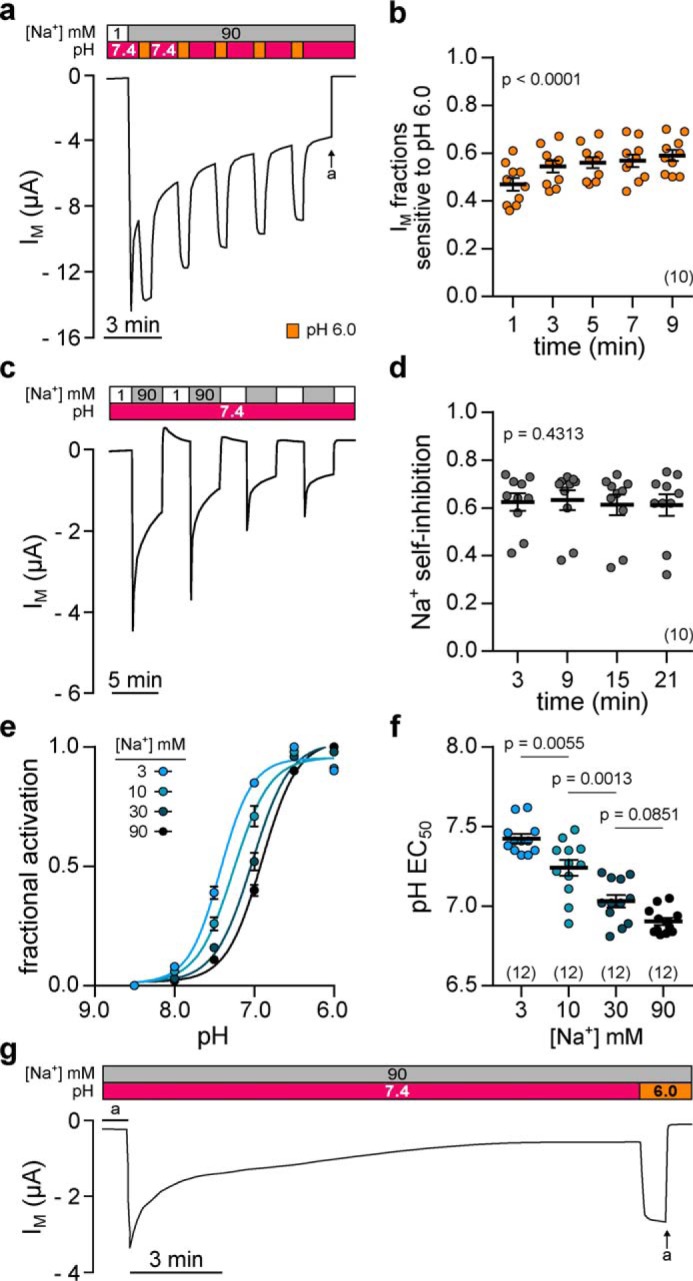
Acidic pH elicits a reversible, consistent, and Na+-dependent activation of Xenopus δβγ-ENaC. a, representative current trace of an oocyte expressing Xenopus δβγ-ENaC. Repetitive acidification of the extracellular milieu (from pH 7.4 to pH 6.0 for 30 s) reversibly increases IM (a = amiloride, 100 μm). b, normalized IM fractions sensitive to pH 6.0 ((IM, pH 6.0 − IM, pH 7.4)/IM, pH 6.0) from recordings as depicted in a. Acid-sensitive current fractions do not decline but rather increase over time (repeated-measures one-way ANOVA, F = 57.8, p < 0.0001; Tukey's multiple comparisons test). c, assessment of repetitive SSI in an oocyte expressing Xenopus δβγ-ENaC. SSI was defined as the decline in IM at 3 min after increasing extracellular [Na+] from 1 to 90 mm. d, Na+ self-inhibition values from recordings as depicted in c. Current fractions lost to SSI ((IM, peak − IM, 3 min)/IM, peak) are constant over time (repeated-measures one-way ANOVA, F = 0.8537, p = 0.4313; Tukey's multiple comparisons test). e, Na+ dependence of acid-induced ENaC activation was examined through determination of the pH EC50 (pH 8.5–6.0; pH 0.5 increments) under different Na+ concentrations (3–90 mm). Fractional channel activation was calculated as the difference between IM at a given pH (IM, x) and the minimal IM (IM, min) in relation to the maximally observed difference in IM (Δmax IM) in the respective recording ((IM, x − IM, min)/Δmax IM)). f, alkaline-shift of the pH EC50 in the presence of reduced [Na+] indicates that less protons are needed for acid-induced channel activation under low [Na+] (one-way ANOVA, F = 38.51, p < 0.0001; Tukey's multiple comparisons test). g, extracellular acidification (from pH 7.4 to pH 6.0) still activates Xenopus δβγ-ENaC after maximal rundown of currents.
