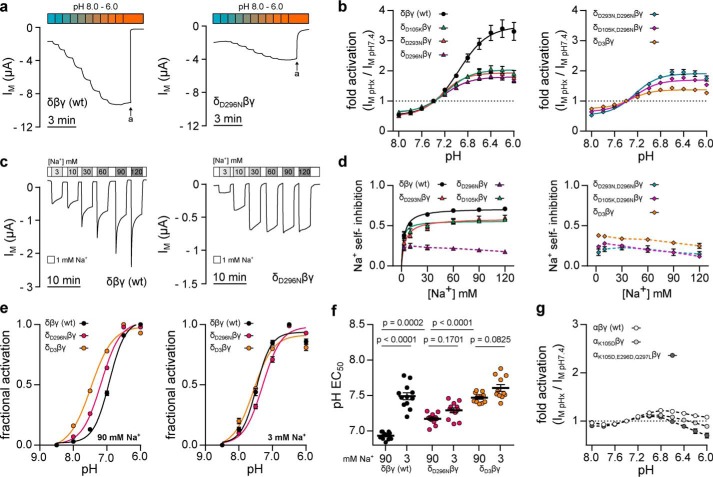
Figure 7.
Three aspartates in the δ-ENaC acidic cleft distinctly affect ENaC sensitivity to pH and Na+. a, representative IM recordings of Xenopus oocytes expressing WT δβγ-ENaC or channels containing a single aspartate to asparagine (δD296Nβγ) mutation. Channel sensitivity to pH was assessed by a stepwise reduction of extracellular pH from 8.0 to 6.0 (pH 0.2 increments). b, pH-dependent activation of δβγ-ENaC containing none (wt), one (δD105Kβγ, δD293Nβγ, and δD296Nβγ), two (δD293N,D296Nβγ and δD105K,D296Nβγ) or three (δD3βγ: δD105K,D293N,D296Nβγ) mutations leading to substitution of single aspartates. Maximum acid-induced channel activation is reduced in ENaC-containing single mutations (δD105Kβγ, δD293Nβγ, and δD296Nβγ) when compared with WT δβγ-ENaC. There is no cumulative effect in channels containing two mutated aspartates, but substitution of three aspartates (δD3βγ) further decreases maximum acid-induced activation as well as alkaline channel inhibition (Kruskal-Wallis test, p < 0.0001; Dunn's multiple comparisons test). c, assessment of SSI at [Na+] from 3 to 120 mm in oocytes expressing WT (δβγ) or mutant (δD296Nβγ) ENaC. d, Na+ self-inhibition ((IM, peak − IM, 3 min)/IM, peak) of WT, single, double, and triple mutant channels. Introduction of δD293N or δD105K moderately decreases maximal SSI when compared with WT δβγ-ENaC, whereas ENaC containing δD296N has a profoundly reduced SSI irrespective of the extracellular [Na+]. Substitution of additional aspartates in double or triple mutant channels does not further decrease ENaC SSI (one-way ANOVA, F = 10.26, p = 0.0003; Tukey's multiple comparisons test). e, proton-sensitivity of δβγ ENaC mutants was assessed by determining fractional channel activation ((IM, x − IM, min)/Δmax IM) resulting from a stepwise reduction of the extracellular pH (pH 8.5–6.0; pH 0.5 increments) in the presence of 90 or 3 mm extracellular Na+. f, reduction of extracellular [Na+] evokes an alkaline-shift of the pH EC50 in WT δβγ-ENaC but not in channels containing the δD296Nβγ or δD3βγ mutations. However, in the presence of 90 mm Na+, introduction of these mutations shifts the pH EC50 to more alkaline values, when compared with WT ENaC (one-way ANOVA, F = 48.02, p < 0.0001; Tukey's multiple comparisons test). g, pH-sensitivity of Xenopus αβγ-ENaC (n = 11) is not enhanced by partial (αK105Dβγ; n = 13) or full (αK105D,E296D,Q297Lβγ; n = 12) reconstitution of the δ-ENaC acidic cleft in this subunit. Individual values for pH-mediated regulation and SSI of WT and mutant ENaC (a–d), including statistical analyses, are listed in Table 1.