Figure 8.
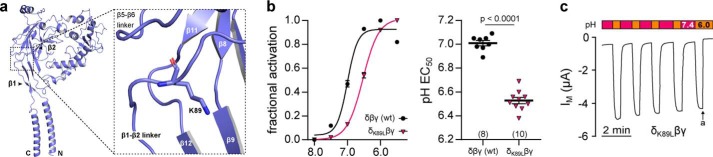
Proton sensitivity of Xenopus δβγ ENaC is further modulated by a lysine in the δ-ENaC β1–β2 linker. a, homology model of the Xenopus δ-ENaC subunit indicating the location of δLys-89 in the linker region between the β1 and β2 sheets in the palm domain. b, fractional channel activation ((IM, x − IM, min)/Δmax IM) resulting from a stepwise reduction of the extracellular pH (pH 8.0–5.5; pH 0.5 increments) in WT and δK89Lβγ ENaC. Introduction of the δK89L mutation leads to a significant acidic-shift of the channel's pH EC50 (Student's unpaired t test). c, representative IM recording of an oocyte expressing δK89Lβγ-ENaC. Macroscopic currents mediated by mutant channels display “stimulus-activated” characteristics as they are low at neutral pH 7.4 and reversibly increased by extracellular acidification (pH 6.0, 30 s; a = amiloride, 100 μm).