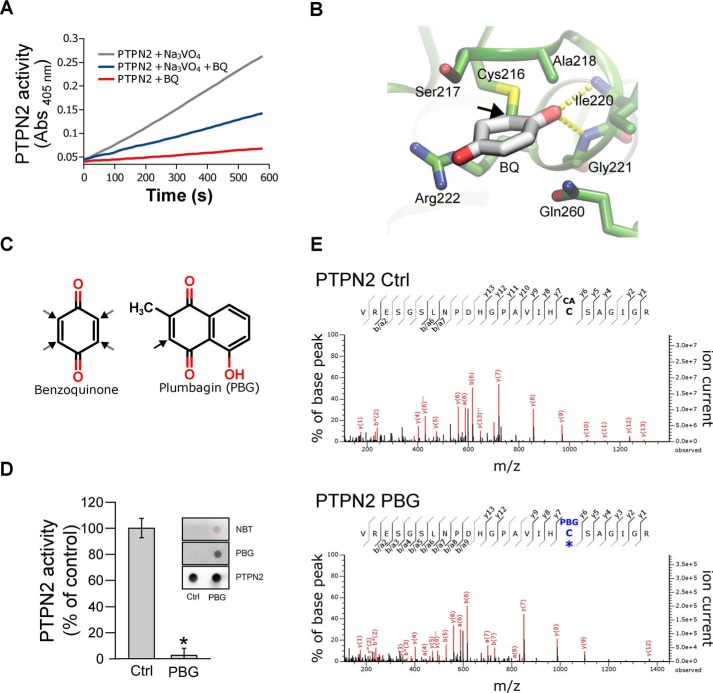
Figure 3.
Irreversible inhibition of PTPN2 by BQ occurs through modification of active-site cysteine. A, recombinant PTPN2 was incubated with BQ (2 μm) for 20 min in the presence or absence of 1 mm orthovanadate (Na3VO4). Progress curves for residual PTPN2 activity were obtained by monitoring pNPP hydrolysis over time (absorbance at 405 nm). B, molecular docking model of BQ bound to human PTPN2 protein structure. BQ adduct atom coordinates were placed inside the PTPN2 active-site pocket as described under “Materials and methods.” BQ is displayed covalently bonded to the Cys-216 residue. C, chemical structures of BQ and PBG. Black arrows, potential Michael adduction sites. D, recombinant PTPN2 was incubated with PBG (50 μm) for 20 min at 37 °C. Residual PTPN2 activity was measured using a pNPP assay. Error bars, S.D. values. *, p < 0.05 compared with control (Ctrl). NBT staining and immunoblotting analysis (using anti-PBG and anti-PTPN2 antibodies) of treated and nontreated samples are shown. E, recombinant PTPN2 was incubated with PBG (100 μm) for 20 min and then incubated with iodoacetamide for 60 min. Samples were separated by SDS-PAGE and analyzed by MS. Mass spectra derived from iodoacetamide or PBG-modified PTPN2 active-site cysteine (Cys-216) peptides are depicted.