Abstract
Background:
Active antibody-mediated rejection (AMR) that occurs during the amnestic response within the first month post-transplant is a rare but devastating cause of early allograft loss after kidney transplant. Prior reports of eculizumab treatment for AMR have been in heterogeneous patient groups needing salvage therapy or presenting at varied time points. We investigated the role of Eculizumab as primary therapy for active AMR early post-transplant.
Methods:
We performed a retrospective observational study of a consecutive cohort of solitary kidney transplant recipients who were transplanted between January 1, 2014 and January 31, 2018 and had AMR within the first 30 days post-transplant and treated with eculizumab ± plasmapheresis.
Results:
Fifteen patients had early active AMR at a median [IQR] of 10 [7-11] days post-transplant and were treated with eculizumab ± plasmapheresis. Thirteen cases were biopsy proven and 2 cases were presumed based on donor specific antibody trends and allograft function. Within 1 week of treatment the median estimated glomerular filtration rate (eGFR) increased from 21 mL/min to 34 mL/min (P = .001); and persistent active AMR was only found in 16.7% (2/12) of biopsied patients within 4-6 months. No graft losses occurred and at last follow-up (median [IQR] of 13 [12-19] months) the median IQR eGFR increased to 52 (46-60) mL/min.
Conclusions:
Prompt eculizumab treatment as primary therapy is safe and effective for early active AMR after kidney transplant or abrupt increases in donor-specific antibodies when biopsy cannot be performed for diagnosis confirmation.
Introduction
Active antibody-mediated rejection (AMR) that occurs during the early memory response within the first month post-transplant is uncommon but can be a devastating cause of early allograft loss if not promptly recognized and treated. Incidence of this pure form of AMR varies from less than 1% in low-risk, minimally sensitized patients with no evidence of preexisting donor-specific antibody (DSA)1,2 to nearly 50% in high-risk patients with a high level of preexisting DSA and a strongly positive crossmatch pretransplant.1,3–5 This condition is thought to occur when memory B cells are activated immediately after transplant, resulting in abrupt increase in preexisting DSAs targeting the allograft.6 Concomitant with the abrupt DSA increase, rapid allograft dysfunction typically occurs for these patients.
The histologic diagnosis of AMR is based on the Banff 2017 classification system and includes histologic evidence of active tissue injury, histologic evidence of antibody interaction with vascular endothelium, and serologic evidence of DSAs.7 Treatment has historically consisted of plasmapheresis or immune adsorption, or both, to reduce serum immunoglobulin and intravenous immunoglobulin (IVIG) for its immunomodulatory effects.8 Adjunctive therapies such as rituximab to target CD20-positive B cells, and bortezomib to decrease plasma cells are used in some cases.9,10 As a last resort, splenectomy can also be considered.11 Even when early AMR is treated successfully, it is associated with chronic AMR and premature allograft loss.4,8,12
Complement activation appears to be a major contributor to tissue damage in the setting of DSA, and thus complement blockade may be an effective AMR treatment.13,14 Eculizumab is a humanized monoclonal antibody that binds to the human C5 complement protein, thereby acting as a terminal complement inhibitor. We have shown previously that eculizumab used preemptively following positive B-cell flow cytometric crossmatch transplant resulted in a reduced incidence of early AMR from 41.0% in historical controls to 7.7% in eculizumab-treated patients.15 Yet, the efficacy of eculizumab as the primary rescue therapy for early active AMR in kidney transplant continues to be unclear. The aim of this study was to examine the outcomes (change in allograft function, allograft loss, and allograft histologic factors) of a consecutive cohort of kidney transplant recipients with early active AMR who were treated with eculizumab.
Methods
We performed a retrospective chart review of consecutive kidney transplant recipients at Mayo Clinic in Rochester, Minnesota, between January 1, 2014, and January 31, 2018, and identified those who had an abrupt increase in DSA and confirmed histologic evidence of AMR within the first 30 days post-transplant (N = 13). We identified 2 additional patients with presumed AMR treated with eculizumab. These 2 (Patients 9, 13) were highly sensitized and had an abrupt increase in DSA but did not undergo a biopsy because of need for anticoagulation. The first patient (Patient 9) underwent a positive crossmatch transplant, had early allograft dysfunction, and an abrupt increase in DSA with prozone detected. Clopidogrel had been started for a recent cardiac stent. The second patient (patient 13) was also highly sensitized with a cPRA of 100%. The crossmatch was negative, but historical DSA was present. This patient was receiving dual anticoagulation for stroke and thrombosis in the clinical setting of lupus anticoagulant. Eculizumab therapy was started at post-operative day 19 when her DSA abruptly increased.
A chart review of electronic health records was performed to obtain basic demographic information, allograft histologic results, allograft function, DSA information, and potential complications from eculizumab treatment.15 The primary outcomes of interest were graft function, allograft survival, and allograft histologic evidence after treatment of early active AMR with eculizumab with or without plasmapheresis and IVIG. This study was approved by the Mayo Clinic Institutional Review Board (18–000921).
Donor Specific Antibody and Flow Cytometric Crossmatch Testing
DSAs were assessed using a solid phase assay (LAB screen, One Lambda, Canoga Park, CA, USA) and cPRA was calculated based on antigens with mean fluorescence index (MFI) >2000 as per center practice. To detect prozone phenomenon, serum was diluted 1:4. Alloantibodies demonstrating prozone were defined as those with a MFI on the diluted/treated sample that was greater than, or equal to the MFI of the same bead on an undiluted sample. All crossmatches were flow cytometric crossmatches.1
Allograft Histologic Analysis
Allograft biopsy tissue was processed for light microscopy and C4d by immunofluorescence (Bio-Rad, Hercules, CA). C4d staining was assessed by indirect immunofluorescence and scored from 0–3. Banff 2017 criteria was used for histologic diagnosis and included 1) histologic evidence of active tissue injury, 2) evidence of antibody interaction with vascular endothelium, and 3) serologic evidence of DSAs.7 Patients in the cohort identified prior to release of Banff 2017 criteria had their biopsy results reviewed to ensure compliance with the latest classification. Biopsies were not performed for 2 patients because of their need for anticoagulation, as described above.
Induction and Maintenance Immunosuppression
Per institution protocol, pre-sensitized patients (DSA with MFI >2000 or positive crossmatch) received induction with anti-thymocyte globulin (1.5 mg/kg per day × 4 doses) and maintenance immunosuppression with tacrolimus, mycophenolate mofetil, and prednisone. Non-sensitized patients received induction with alemtuzumab (20 mg × 1 dose) and maintenance immunosuppression with tacrolimus and mycophenolate mofetil.
Identification and Treatment of Early AMR
At the time of DSA increase suggestive of AMR, an urgent allograft biopsy was performed and eculizumab (1200 mg) was given within 24 hours. Patients with allograft dysfunction (either decrease in estimated glomerular filtration rate [eGFR] or delayed graft function) also started treatment with plasmapheresis or IVIG, or both. Plasmapheresis was initiated within 24 to 48 hours of diagnosis. Eculizumab treatment (600 mg intravenous) was repeated after each plasmapheresis treatment. If plasmapheresis was discontinued and it was deemed by the treating physician that eculizumab treatment should be continued, a 900-mg dose was repeated weekly. Eculizumab treatment was stopped after the DSA MFI decreased to 500016,17 or the patient had received eculizumab for 30 days with stabilization of renal function. All patients who received eculizumab were prescribed oral penicillin or ciprofloxacin for infectious disease prophylaxis in addition to standard post-transplant infectious disease prophylaxis. All patients were also advised to receive meningococcal vaccine.
The number of plasmapheresis treatments varied on the basis of clinical context and our experience with using eculizumab. At the beginning of our experience, patients received up to 12 plasmapheresis treatments. After finding that most patients stabilized clinically after the initial dose of eculizumab, we began to withhold plasmapheresis or decrease the number of treatments per patient when the allograft function stabilized. Each patient received 100 mg/kg of IVIG after each plasmapheresis treatment and 1 g/kg of IVIG at the end of all plasmapheresis treatments.
Statistical computation was performed on SPSS software version 20.0 (IBM Corp). Medians and interquartile ranges (IQRs) were reported. Nonparametric continuous variables were compared using the matched pairs analysis. P values less than .05 were considered statistically significant.
Results
Baseline Demographic Characteristics
The incidence of early active AMR from January 1, 2014, through January 31, 2018, was 1.8% (15/850). The median age of patients at the time of AMR was 51 years, and the majority of patients were female and white (Table 1). Approximately one-half of the patients received a transplant from a living donor. Twelve (80.0%) patients had evidence of detectable DSA using single antigen bead testing (LABScreen, One Lambda Conoga Park, CA), and six (40.0%) patients had a positive B flow cytometric crossmatch at baseline. Among the patients with a positive B-cell flow cytometric crossmatch at baseline, the median mean channel shift was 149.5. The median (IQR) cPRA was 93% (0%−99%). Of the 10 female patients, 8 (80.0%) had a prior pregnancy, and 7 (46.7%) of the 15 patients had prior history of kidney transplant. Of the 8 females with a prior pregnancy, 5 (62.5%) received kidney transplants were from a living donor, and only 1 (12.5%) of them received a kidney from a spouse that was a father of a child. Of the 7 patients with prior kidney transplant(s), we only had details on 2 patients’ prior donor HLA typing, with the rest having been transplanted elsewhere in the remote past. These 2 patients did not have HLA mismatches to antigens common to both the prior and current donor.
Table 1.
Demographic Characteristics of the 15 Study Patients
| Overall Cohortc | |
|---|---|
| Age, median (IQR), y | 51 (42-55) |
| Female gender | 10 (66.7) |
| Ethnicity | |
| White | 11 (73.3) |
| African American | 2 (13.3) |
| Asian | 2 (13.3) |
| Primary cause of ESRD | |
| Diabetes mellitus | 1 (6.7) |
| Cystic disease | 5 (33.3) |
| Glomerulonephritis | 4 (26.7) |
| Unknown | 1 (6.7) |
| Others | 4 (26.7) |
| Type of donor | |
| Living | 8 (53.3) |
| Deceased | 7 (46.7) |
| BMI, median (IQR), kg/m2 | 29.4 (26.2-36.5) |
| Blood group | |
| O | 4 (26.7) |
| A | 9 (60.0) |
| B | 1 (6.7) |
| AB | 1 (6.7) |
| Baseline detectable DSA (any MFI) | 12 (80.0) |
| Baseline positive B flow cytometric XM | 6 (40.0) |
| Baseline channel shift among +XM, median (IQR) (n = 6) | |
| B | 149.5 (113.3-187.5) |
| T | 89 (48.25-128.5) |
| Baseline sum MFI of DSA procedures among +XM, median (IQR) (n = 6) | |
| Class I | 1507 (0-2727) |
| Class II | 0 (0-2347.5) |
| Receiving dialysis at time of transplant | 9 (60.0) |
| cPRA, median (IQR) | 93 (0-99) |
| Prior pregnancy (n = 10) | 8 (80.0) |
| Retransplant | 7 (46.7) |
| HLA mismatches, median (IQR) | |
| A, B, DR | 4 (3-5) |
| Class II DR, DP | 2 (1-4) |
| Preconditioning with rituximab and plasmapheresis | 1 (6.7) |
| Type of induction | |
| Thymoglobulin | 10 (66.7) |
| Alemtuzumab | 5 (33.3) |
| Initial maintenance immunosuppression | |
| Tacrolimus, mycophenolate mofetil, prednisone | 10 (66.7) |
| Tacrolimus, mycophenolate mofetil | 5 (33.3) |
| Delayed graft function | 3 (20.0) |
| Follow-up, median (IQR), months | 13(12-19) |
Abbreviations: BMI, body mass index; cPRA, calculated panel-reactive antibody; DSA, donor-specific antibody; ESRD, end-stage renal disease; IQR, interquartile range; MFI, mean fluorescence intensity; XM, crossmatch; +XM, positive crossmatch.
Values are presented as number and percentage of patients unless specified otherwise.
Only 1 patient received preemptive rituximab treatment and plasmapheresis before an intended positive crossmatch transplant. The majority [66.6%(10/15)] of patients had detectable DSA prior to transplant and received induction with anti-thymocyte globulin, and maintenance immunosuppression with tacrolimus, mycophenolate mofetil, and prednisone. The other 5 patients (33.3%) received induction with alemtuzumab and received initial maintenance immunosuppression with tacrolimus and mycophenolate mofetil. After the AMR episode, all patients received maintenance immunosuppression that included prednisone (5 mg). Median follow-up was 13 months.
AMR Diagnosis and Treatment Course
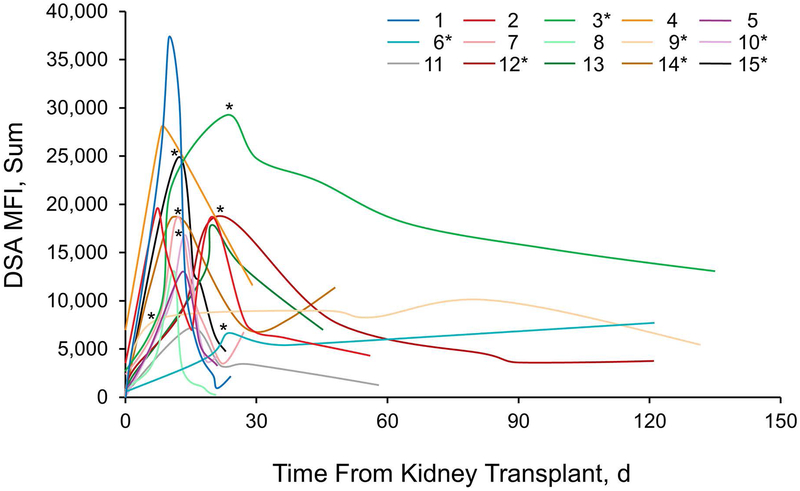
AMR was diagnosed a median (IQR) of 10 (7–11) days post-transplant, at which time the median (IQR) eGFR was 21 (12–32) mL/min and median (IQR) creatinine was 2.4 (1.8–4.4) mg/dL (Table 2). The median (IQR) of class I DSA MFI was 7196 (1165–9396) and the median (IQR) of class II DSA MFI was 4808 (0–9798) at the time of AMR diagnosis. Serum dilutions revealed that 46.7% (7/15) of patients had prozone at the time of AMR diagnosis (Figure 1). All the patients were treated with eculizumab within 24 hours of presumptive diagnosis of AMR. Biopsies were performed at AMR onset in 86.7% (13/15) of patients.
Table 2.
AMR Details and Treatment (N=15)
| AMR and Treatment | Value |
|---|---|
| Post-transplant day, median (IQR) | 10 (7-11) |
| Serum creatinine at onset of AMR, median (IQR), mg/dL | 2.4 (1.8-4.4) |
| eGFR at onset of AMR, median (IQR), mL/min | 21 (12-32) |
| Doses of eculizumab, median (IQR), No. | 5 (2-10) |
| Plasmapheresis, No. (%) | 12 (80.0) |
| Sessions of plasmapheresis, median (IQR), No. | 7 (3-10) |
| Splenectomy, No. (%) | 1 (6.7) |
Abbreviations: AMR, antibody-mediated rejection; eGFR, estimated glomerular filtration rate; IQR, interquartile range.
Figure 1.
DSA MFI Levels at Time of Antibody-Mediated Rejection (AMR) Diagnosis, and Follow-up. Numbers indicate individual patients. Asterisk indicates that serial dilutions of patient’s serum showed prozone effect at peak of AMR response. DSA indicates donor-specific antibody; MFI, mean fluorescence intensity.
The median number of treatment doses of eculizumab was 5. Of the 15 patients, 12 (80.0%) received at least 1 plasmapheresis treatment, and the median number of plasmapheresis treatments was 7 (Table 2). Patients who had plasmapheresis had more doses of eculizumab than those who did not have plasmapheresis (median, 8.5 doses [vs 2 doses]; P = .048). One patient was treated with splenectomy (performed on post-operative day 12) because of oliguria, allograft dysfunction, and persistently high DSA.
All biopsies met Banff 2017 criteria for AMR. Specifically, all (13/13) showed diffuse C4d positivity in the peritubular capillaries of ≥2; 30.8% (4/14) showed peritubular capillaritis ≥2; 15.4% (2/13) showed glomerulitis ≥2; and 100.0% (13/13), acute tubular injury (Table 3). None of the biopsies showed evidence of thrombotic microangiopathy or cortical necrosis. Of note, there were 5 (38.5%) patients (of the 13 biopsied) that had positive C4d staining and acute tubular injury, without evidence of glomerulitis or peritubular capillaritis. These patients had time zero biopsies that showed nil or minimal acute tubular injury.
Table 3.
Allograft Histologic Summary
| Biopsya |
|||
|---|---|---|---|
| Diagnosis and Banff 2017 Scores | Index (n = 13) |
Follow-up 3-6 months (n = 12) |
Follow-up 12 months (n = 10) |
| Primary diagnosis | |||
| Isolated T-cell–mediated rejection | 0 (0.0) | 2 (16.7)b | 1 (10)d |
| Isolated active AMR | 13 (100.0) | 0 (0.0) | 2 (20) |
| Isolated chronic active AMR | 0 (0.0) | 0 (0.0) | 1 (10) |
| Mixed active AMR and T-cell–mediated rejection | 0 (0.0) | 2 (16.7)c | 0 (0.0) |
| No rejection | 0 (0.0) | 8 (66.7) | 6 (60) |
| Banff 2017 (score) | |||
| C4d positivity (≥2) | 13 (100.0) | 1 (8.3) | 1 (10) |
| Peritubular capillaritis (≥2) | 4 (30.8) | 2 (16.7) | 2 (20) |
| Glomerulitis (≥2) | 2 (15.4) | 2 (16.7) | 2 (20) |
| Chronic glomerulopathy (≥1) | 0 (0.0) | 1 (8.3) | 1 (1) |
Abbreviation: AMR, antibody-mediated rejection.
Two patients did not undergo biopsy because of need for anticoagulation and 1 patient did not undergo biopsy at 1 year because bowel was overlying allograft on ultrasound. The other biopsies not included were either not performed because of patient preference or patient has not reached time point.
Grades 1A and 1B
Borderline and Grade 1A
Borderline
Outcomes Following AMR Treatment
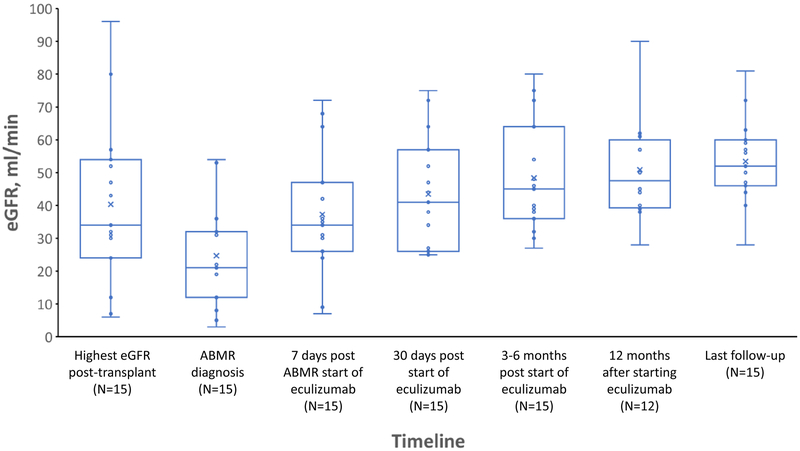
No graft losses occurred during follow-up [median (IQR) follow-up, 13(12–19) months]. Within 1 week of treatment, the median (IQR) eGFR increased from 21 mL/min to 34 mL/min (IQR, 26–47 mL/min), P = .001. The median (IQR) eGFR had further increased to 41 (26–47) mL/min, P = .01 after 1 month of treatment, and at 3 to 6 months post-treatment the eGFR was 45 (36–64) mL/min. Twelve patients reached the 12 months post-transplant time point at which time the median (IQR) eGFR was 45 (41.5–59.0) mL/min. The median (IQR) eGFR at the point of last follow-up [median(IQR) of 13(12–19) months] was 52 (46–60) mL/min. (Figure 2).
Figure 2.
Change in eGFR After AMR Diagnosis. Within 1 week of eculizumab treatment the median estimated glomerular filtration rate (eGFR) increased from 21 mL/min to 34 mL/min (P = .001) and up to 41 mL/min within 1 month of treatment (P = .01). AMR indicates antibody-mediated rejection; eGFR, estimated glomerular filtration rate. Matched pairs analysis was used to compare median eGFRs.
Follow-up surveillance biopsies were performed in 80.0% (12/15) of patients at the 3 to 6 month time point. Again, the 2 patients who did not have index biopsies because of the need for anticoagulation did not have follow-up protocol biopsies. Only 16.7% (2/12) of patients had histologic evidence of active AMR based on Banff 2017 criteria, and both these patients had a concomitant T-cell–mediated rejection. One patient had Banff IA (i2, t2, v0) while the other had borderline rejection (i1, t1, v0). Isolated T-cell–mediated rejection was identified in 16.7% (2/12) of these patients – one patient had Banff IA (i2, t2, v0) while the other had Banff IB (i3, t3, v0). The remaining 66.6% (8/12) of patients had no evidence of rejection. Both patients with evidence of active AMR had glomerulitis (g≥2) and peritubular capillaritis (ptc ≥2). Only 1 patient had ongoing C4d positivity. This patient also had evidence of chronicity with a Banff cg score of 2. Ten patients (66.7%) had a surveillance biopsy at 1 year after AMR treatment: 20.0% (2/10) patients had isolated active AMR; 10.0% (1/10) had chronic active AMR; and 10.0% (1/11) had an isolated borderline acute T-cell–mediated rejection. Six patients (60.0%) had no histologic evidence of rejection (Table 3). Withholding plasmapheresis was not associated with AMR. The 2 patients who did not receive plasmapheresis and had a follow-up kidney biopsy at 3 to 6 months did not have evidence of persistent AMR. Both patients with active AMR on follow-up biopsy received plasmapheresis (8 and 12 treatments, respectively).
One patient had Haemophilus influenzae pneumonia at 2.5 months after discontinuation of eculizumab treatment. No other complications associated with eculizumab were identified. Specific details of each patient’s clinical course and biopsy results are shown in Table 4.
Table 4.
Individual Cases of AMR
| Onset of AMRa | Follow-up Biopsya | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient No. |
DGF | POD | Cr, mg/dL |
eGFR, mL/min |
Banff 2017 Score | DSAb | Prozone Detected |
Eculizumab Rx | PLEX, No. |
At 4-6 mo | At 12mo | ||||||||||||
| G | I | T | V | PTC | C4d | Other | Class I |
Class II |
Doses, No. |
Cumulative Dose, mg |
Cr, mg/dL |
eGFR, mL/min |
Biopsy Result |
Cr, mg/dL |
eGFR, mL/min |
Biopsy Result |
|||||||
| 1 | No | 7 | 4.4 | 12 | 1 | 0 | 0 | 0 | 3 | 2 | ATI | 12 184 | 12 779 | No | 9 | 6300 | 10 | 1.3 | 48 | No rejection | 1.4 | 44 | No rejection |
| 2 | No | 7 | 1.8 | 53 | 0 | 0 | 0 | 0 | 0 | 3 | ATI | 19 101 | 0 | No | 11 | 7200 | 12 | 1.1 | 75 | No rejection | 1.3 | 61 | T-cell–mediated rejection (borderline) |
| 3 | No | 8 | 3.6 | 19 | 1 | 0 | 0 | 0 | 2 | 3 | ATI | 9396 | 1780 | Yes | 31 | 30 600 | 12 | 1.9 | 39 | Mixed active AMR and T-cell–mediated rejection (IA) | 1.9 | 39 | CAMR |
| 4 | No | 8 | 1.5 | 36 | 0 | 0 | 0 | 0 | 0 | 3 | ATI | 0 | 27 645 | No | 9 | 6300 | 8 | 1.2 | 46 | – | 1.0 | 57 | Active AMR |
| 5 | No | 10 | 2.5 | 21 | 0 | 0 | 0 | 0 | 1 | 3 | ATI | 1165 | 9798 | No | 5 | 3600 | 7 | 1.7 | 32 | Mixed Active AMR and borderline acute cellular rejection | 1.5 | 45 | No rejection |
| 6 | No | 19 | 2.6 | 32 | 0 | 0 | 0 | 0 | 2 | 3 | ATI | 4156 | 0 | Yes | 2 | 2100 | 2 | 1.8 | 40 | No rejection | 1.6 | 50 | Active AMR |
| 7 | No | 11 | 2.4 | 32 | 2 | 1 | 0 | 2 | 3 | 3 | ATI | 8054 | 431 | No | 8 | 5400 | 8 | 1.2 | 80 | No rejection | 1.5 | 62 | No rejection |
| 8 | No | 11 | 1.7 | 31 | 2 | 0 | 0 | 0 | 1 | 3 | ATI | 13 061 | 0 | No | 1 | 1200 | 1 | 1.6 | 27 | No rejection | 1.9 | 28 | No biopsy performedc |
| 9 | Yes | 7 | 11.5 | 3 | – | – | – | – | – | – | – | 601 | 7347 | Yes | 11 | 8400 | 7 | 1.5 | 36 | – | 1.7 | 40 | – |
| 10 | No | 10 | 2.4 | 21 | 0 | 0 | 0 | 0 | 0 | 3 | ATI | 9283 | 0 | Yes | 4 | 3000 | 4 | 0.9 | 72 | No rejection | .8 | 90 | No rejection |
| 11 | No | 15 | 2.2 | 22 | 0 | 0 | 0 | 0 | 0 | 3 | ATI | 7196 | 0 | No | 1 | 1200 | 0 | 1.2 | 45 | T-cell–mediated rejection (IA) | .9 | 57 | No rejection |
| 12 | Yes | 10 | 7.5 | 8 | 0 | 0 | 0 | 0 | 1 | 3 | ATI | 522 | 8352 | Yes | 4 | 3900 | 0 | 1.9 | 38 | T-cell–mediated rejection (IB) | 1.9 | 38 | No rejection |
| 13 | No | 20 | 1.1 | 54 | – | – | – | – | – | – | – | 7448 | 5028 | No | 2 | 2400 | 0 | 1.2 | 64 | – | – | – | – |
| 14 | No | 10 | 2.4 | 21 | 1 | 0 | 0 | 0 | 0 | 3 | ATI | 2955 | 15 201 | Yes | 10 | 6900 | 3 | 1.8 | 30 | No rejection | – | – | – |
| 15 | Yes | 11 | 9.5 | 5 | 0 | 0 | 0 | 0 | 0 | 3 | ATI | 19 812 | 4808 | Yes | 5 | 3600 | 4 | 1.3 | 54 | No rejection | – | – | – |
Abbreviations: AMR, antibody-mediated rejection; ATI, acute tubular injury; CAMR, chronic active antibody-mediated rejection; Cr, creatinine; DGF, delayed graft function; DSA, donor-specific antibody; eGFR, estimated glomerular filtration rate; MFI, mean fluorescence intensity; PLEX, plasma exchange; POD, post-operative day.
Dash indicates not applicable or not available.
Sum MFI.
No biopsy performed because bowel was overlying allograft on ultrasound.
Discussion
We found that administering eculizumab alone or in combination with plasmapheresis and IVIG was a safe, rapid, and effective means of therapy for early active AMR and abrupt DSA increases that can occur during robust amnestic response early after transplant. Allograft function improved significantly within 1 week of treatment with a median increase in eGFR from 21 mL/min to 34 mL/min (P = .001). At last follow-up, the median eGFR had increased to 52 mL/min and no patients had allograft loss. In addition, the majority patients had complete resolution of histologic evidence of AMR within 3 to 6 months.
Previously, treatments with eculizumab or other complement inhibitors for AMR have been reported for small and heterogeneous patient groups that presented at varied times post-transplant, and success of treatment has been mixed.11,18–21 Two reports in kidney transplant have shown that eculizumab combined with IVIG and plasmapheresis was ineffective for treating active AMR.22,23 In another case series, 80% (4/5) of positive crossmatch kidney transplant recipients who had early clinical AMR and were treated with a combination of eculizumab, plasmapheresis, and IVIG lost their grafts at a median of 95 days post-transplant.11 The lack of success of eculizumab therapy for AMR in these small case reports is unclear but is likely related to the timing of eculizumab administration, the severity and chronicity of AMR at treatment initiation, and the baseline patient profile (ie, positive crossmatch).
Our cohort was distinctive from others because of its size and its homogeneity because we studied only patients with early active AMR within the first month after transplant, and eculizumab was used early (within 24 hours of AMR diagnosis or identification of increased DSA) rather than as salvage therapy. We also have follow-up biopsies on the majority of patients at the 3 to 6 month time point.
The main limitations of our study were the lack of a comparator group, the non-standardized and heterogenous treatment regimens, and the lack of histologic information on all of the patients at all time studied time points. One problem is that randomized controlled trials to answer such questions are difficult to perform. Early active AMR is rare (1.8% in a 4-year period), and it would be difficult to obtain adequate enrollment within a reasonable time frame with correct characterization of patient profiles in a multicenter study. This issue is well illustrated in a previous randomized controlled trial comparing eculizumab to standard of care for AMR (ClinicalTrials.gov Identifier: NCT01895127). That study was terminated early for lack of efficacy, but only 11 patients were enrolled and the timing of AMR was not specified. The use of a historical control group is also problematic because of the small sample size and differences in clinical practice long term.
We also want to emphasize that the AMR in this patient cohort was detected very early and may have been a milder form because 53.8% (7/13) of the patients studied had minimal microvascular inflammation (g = 0, ptc = 0, or g + ptc = <2). Lastly, we note that among those with a positive B-cell flow cytometric crossmatch, the median channel shift of 149.5 was less than the threshold of more than 359 identified by Burns et al.16 and thus should have had a lower propensity for AMR.
Given that our case series was small and non-randomized, we cannot conclude that eculizumab treatment is superior to standard-of-care therapies, including plasmapheresis, IVIG, or rituximab, for AMR treatment. However, it is an effective therapeutic option for AMR that has clear advantages compared with other therapies. Eculizumab is easy to administer, is well tolerated with minimal adverse effects, and blocks terminal complement immediately. It has rapid effectiveness that can prevent early graft loss, and invasive therapies such as plasmapheresis that are associated with adverse effects (ie, infection, electrolyte abnormalities, and hemorrhage from depletion of coagulation factors) can sometimes be avoided.24,25 Use of eculizumab is also easier for the patient because it can usually be administered as an outpatient without a central venous catheter.
The addition of plasmapheresis is theoretically advantageous because it is particularly effective at removing immunoglobulin M, which has been associated with AMR in the clinical setting of terminal complement blockade.26 The drawback is that plasmapheresis does not affect long-term alloantibody production, and some previous studies have suggested that alloantibody eventually decreases on its own after the early amnestic response anyway.27 The other problem with plasmapheresis is that it removes eculizumab and therefore eculizumab must be redosed, which increases the overall cost of therapy dramatically.
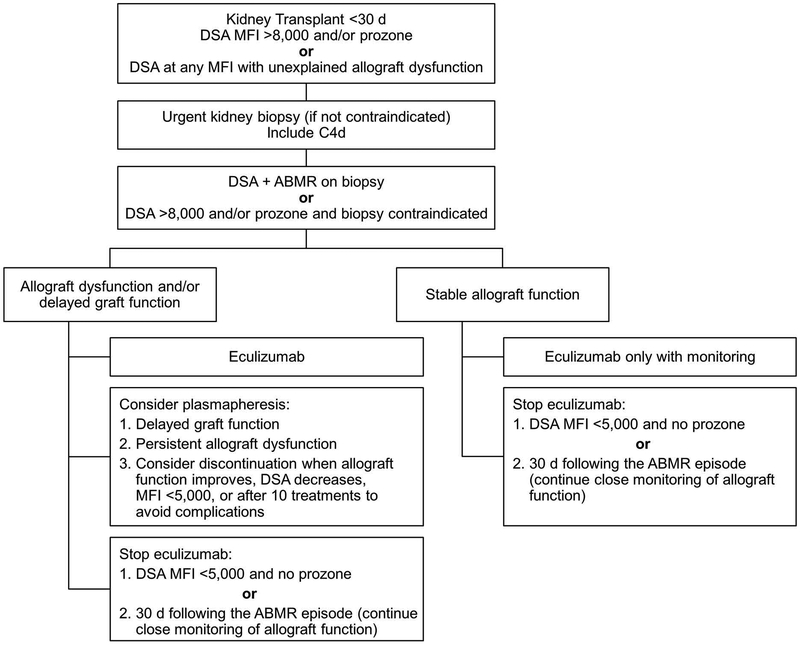
On the basis of our observations and prior experience,12,15,16,28 we have developed a treatment algorithm using eculizumab to manage early abrupt increases in DSA and histologic evidence of AMR within 30 days after kidney transplant (Figure 3). We recommend urgent kidney biopsy with C4d immunostaining whenever possible for patients with 1) an early (<30 days post-transplant) increase in DSA with an MFI greater than 8000; 2) evidence of prozone effect detected with serum dilution; or 3) DSAs present at any level with unexplained allograft dysfunction. For patients who meet histologic criteria for AMR or cannot undergo a biopsy because of risks, we recommend initiation of eculizumab therapy. In patients who have allograft dysfunction (either a severe decrease in eGFR or delayed graft function), we recommend considering plasmapheresis and IVIG. We believe that it is safe for patients with stable allograft function to receive eculizumab alone with close monitoring. In both scenarios, we recommend discontinuing eculizumab therapy when the DSA MFI is less than 5000 and prozone is no longer present or at 30 days following treatment initiation regardless of DSA level. We recommend only a limited course of eculizumab therapy because of our previous finding that long-term eculizumab treatment does not prevent chronic AMR.12 The choice of discontinuing eculizumab at DSA MFI of 5000 is based on our groups prior published experience that DSA with an MFI of 5000 is typically not associated with AMR.16
Figure 3.
Proposed Algorithm for Managing Early AMR and Increases in DSA Early After Kidney Transplant. AMBR indicates antibody-mediated rejection; DSA, donor-specific antibody; MFI, mean fluorescence intensity.
The main disadvantage for eculizumab use is its high cost and limited availability. We believe that the financial cost can be justified when considering the full “cost” of a graft loss. Patients with early active AMR, particularly those with significant allograft dysfunction and histologic injury, are historically at high risk for graft loss if they are not treated promptly and aggressively. Early allograft loss is devastating to the patient, leading to the resumption of dialysis (which itself is costly). Performing a retransplant for these patients can become a challenge because of subsequent sensitization.
Another understated cost is the impact of a graft loss to a transplant program. Thus, the cost of eculizumab must be considered in the context of the other costs of AMR treatment, including central line placement, plasmapheresis, IVIG, hospitalizations, and other adjunctive therapies. All these therapies are expensive and have potential adverse effects. The patient is also inconvenienced by having to stay close to an inpatient facility for the AMR treatments, while eculizumab can be safely administered in a basic facility. The other major consideration apart from cost is prompt availability of eculizumab. Delayed treatment of an aggressive early AMR can lead to graft loss and allograft damage that are not easily reversible. We believe that part of our success was related to the fact that eculizumab is readily available at our center and can be given promptly.
Our experience lies in using eculizumab therapy, which inhibits terminal complement, but it is possible that other complement inhibitors are also effective. Previous preliminary studies have suggested that C1 esterase inhibitors can also be used to treat AMR, and randomized controlled trials are underway using them for refractory active AMR and prevention of chronic AMR (ClinicalTrials.gov Identifier: NCT03221842 and ClinicalTrials.gov Identifier: NCT02547220).
In summary, prompt use of eculizumab appears to be a safe and effective therapy for treating early active AMR or abrupt increases in DSA when a biopsy cannot be performed safely in kidney transplant recipients at less than 30 days post-transplant. Eculizumab appears to prevent devastating early graft loss with minimal adverse effects, but it is unclear whether this strategy decreases the long-term incidence of chronic AMR. Further study of therapy duration, role of concomitant plasmapheresis, cost-effectiveness, and comparison with other complement inhibitor therapies is needed, as well as treatment to modify alloimmune responses.
Acknowledgments
This publication was supported by Clinical and Translational Science Awards Grant Number KL2 TR002379 from the National Center for Advancing Translational Sciences (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- AMR
antibody-mediated rejection
- cPRA
calculated panel-reactive antibody
- DSA
donor-specific antibody
- eGFR
estimated glomerular filtration rate
- IQR
interquartile range
- IVIG
intravenous immunoglobulin
- MFI
mean fluorescence intensity
References
- 1.Schinstock CA, Gandhi M, Cheungpasitporn W, et al. Kidney Transplant With Low Levels of DSA or Low Positive B-Flow Crossmatch: An Underappreciated Option for Highly Sensitized Transplant Candidates. Transplantation. 2017;101(10):2429–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sadaka B, Ejaz NS, Shields AR, et al. A Banff Component Scoring-based Histologic Assessment of Bortezomib-based Antibody-mediated Rejection Therapy. Transplantation. 2015;99(8):1691–1699. [DOI] [PubMed] [Google Scholar]
- 3.Crespo M, Pascual M, Tolkoff-Rubin N, et al. Acute humoral rejection in renal allograft recipients: I. Incidence, serology and clinical characteristics. Transplantation. 2001;71(5):652–658. [DOI] [PubMed] [Google Scholar]
- 4.Orandi BJ, Chow EH, Hsu A, et al. Quantifying renal allograft loss following early antibody-mediated rejection. Am J Transplant. 2015;15(2):489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gloor JM, DeGoey SR, Pineda AA, et al. Overcoming a positive crossmatch in living-donor kidney transplantation. Am J Transplant. 2003;3(8):1017–1023. [DOI] [PubMed] [Google Scholar]
- 6.Schinstock C, Stegall MD. Acute Antibody-Mediated Rejection in Renal Transplantation: Current Clinical Management. Curr Transplant Rep. 2014;1(2):78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haas M, Loupy A, Lefaucheur C, et al. The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 2018;18(2):293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lefaucheur C, Nochy D, Andrade J, et al. Comparison of combination Plasmapheresis/IVIg/anti-CD20 versus high-dose IVIg in the treatment of antibody-mediated rejection. Am J Transplant. 2009;9(5):1099–1107. [DOI] [PubMed] [Google Scholar]
- 9.Vo AA, Peng A, Toyoda M, et al. Use of intravenous immune globulin and rituximab for desensitization of highly HLA-sensitized patients awaiting kidney transplantation. Transplantation. 2010;89(9):1095–1102. [DOI] [PubMed] [Google Scholar]
- 10.Waiser J, Budde K, Schütz M, et al. Comparison between bortezomib and rituximab in the treatment of antibody-mediated renal allograft rejection. Nephrol Dial Transplant. 2012;27(3):1246–1251. [DOI] [PubMed] [Google Scholar]
- 11.Orandi BJ, Zachary AA, Dagher NN, et al. Eculizumab and splenectomy as salvage therapy for severe antibody-mediated rejection after HLA-incompatible kidney transplantation. Transplantation. 2014;98(8):857–863. [DOI] [PubMed] [Google Scholar]
- 12.Cornell LD, Schinstock CA, Gandhi MJ, Kremers WK, Stegall MD. Positive crossmatch kidney transplant recipients treated with eculizumab: outcomes beyond 1 year. Am J Transplant. 2015;15(5):1293–1302. [DOI] [PubMed] [Google Scholar]
- 13.Montgomery RA, Orandi BJ, Racusen L, et al. Plasma-Derived C1 Esterase Inhibitor for Acute Antibody-Mediated Rejection Following Kidney Transplantation: Results of a Randomized Double-Blind Placebo-Controlled Pilot Study. Am J Transplant. 2016;16(12):3468–3478. [DOI] [PubMed] [Google Scholar]
- 14.Viglietti D, Gosset C, Loupy A, et al. C1 Inhibitor in Acute Antibody-Mediated Rejection Nonresponsive to Conventional Therapy in Kidney Transplant Recipients: A Pilot Study. Am J Transplant. 2016;16(5):1596–1603. [DOI] [PubMed] [Google Scholar]
- 15.Stegall MD, Diwan T, Raghavaiah S, et al. Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am J Transplant. 2011;11(11):2405–2413. [DOI] [PubMed] [Google Scholar]
- 16.Burns JM, Cornell LD, Perry DK, et al. Alloantibody levels and acute humoral rejection early after positive crossmatch kidney transplantation. Am J Transplant. 2008;8(12):2684–2694. [DOI] [PubMed] [Google Scholar]
- 17.Loethen A, Lichvar A, Tremblay S, et al. Clinical Heterogeneity of Early Anamnestic Donor Specific Antibody Responses in Kidney Transplantation. Am J Transplant. 2017;17 (suppl 3). [Google Scholar]
- 18.Muller YD, Aubert JD, Vionnet J, et al. Acute antibody-mediated rejection one week after lung transplantation successfully treated with eculizumab, intravenous immunoglobulins and rituximab. Transplantation. 2018;102(6):e301–e303. [DOI] [PubMed] [Google Scholar]
- 19.Biglarnia AR, Nilsson B, Nilsson T, et al. Prompt reversal of a severe complement activation by eculizumab in a patient undergoing intentional ABO-incompatible pancreas and kidney transplantation. Transpl Int. 2011;24(8):e61–e66. [DOI] [PubMed] [Google Scholar]
- 20.Fan J, Tryphonopoulos P, Tekin A, et al. Eculizumab Salvage Therapy for Antibody-Mediated Rejection in a Desensitization-Resistant Intestinal Re-Transplant Patient. Am J Transplant. 2015;15(7):1995–2000. [DOI] [PubMed] [Google Scholar]
- 21.Locke JE, Magro CM, Singer AL, et al. The use of antibody to complement protein C5 for salvage treatment of severe antibody-mediated rejection. Am J Transplant. 2009;9(1):231–235. [DOI] [PubMed] [Google Scholar]
- 22.Burbach M, Suberbielle C, Brochériou I, et al. Report of the inefficacy of eculizumab in two cases of severe antibody-mediated rejection of renal grafts. Transplantation. 2014;98(10):1056–1059. [DOI] [PubMed] [Google Scholar]
- 23.Yelken B, Arpali E, Görcin S, et al. Eculizumab for Treatment of Refractory Antibody-Mediated Rejection in Kidney Transplant Patients: A Single-Center Experience. Transplant Proc. 2015;47(6):1754–1759. [DOI] [PubMed] [Google Scholar]
- 24.Gubensek J, Buturovic-Ponikvar J, Kandus A, et al. Treatment of Antibody-Mediated Rejection After Kidney Transplantation - 10 Years’ Experience With Apheresis at a Single Center. Ther Apher Dial. 2016;20(3):240–245. [DOI] [PubMed] [Google Scholar]
- 25.Youngblood SC, Deng Y, Chen A, Collard CD. Perioperative therapeutic plasmapheresis. Anesthesiology. 2013;118(3):722–728. [DOI] [PubMed] [Google Scholar]
- 26.Bentall A, Tyan DB, Sequeira F, et al. Antibody-mediated rejection despite inhibition of terminal complement. Transpl Int. 2014;27(12):1235–1243. [DOI] [PubMed] [Google Scholar]
- 27.Schinstock CR, Cosio F, Cornell L, Gandhi M, Everly M, Stegall M. Donor-Specific Alloantibody after Positive Crossmatch Kidney Transplant with Eculizumab (EC): Correlation with Transplant Glomerulopathy and Graft Failure [Abstract]. Am J Transplant. 2015;15(suppl 3). [Google Scholar]
- 28.Gloor JM, Winters JL, Cornell LD, et al. Baseline donor-specific antibody levels and outcomes in positive crossmatch kidney transplantation. Am J Transplant. 2010;10(3):582–589. [DOI] [PubMed] [Google Scholar]