Abstract
Irreversible electroporation (IRE) is a relatively recent method of ablation. In contrast to many ablation devices that use thermal methods to induce cell death, IRE employs the use of an electric field to cause irreversible permeability of the cell membrane, thus inducing apoptosis. Since its use in the pancreas was first described in 2012, IRE has become established as part of the armamentarium of ablation devices currently available. The crucial advantage of IRE compared with other devices employing thermal ablation is the safety around vital structures such as vessels and ducts. This is especially important in the pancreas due to the close proximity of multiple major vascular structures, biliary ducts, and adjacent gastrointestinal organs. This article will explore the current evidence regarding the use of IRE in the pancreas.
Keywords: interventional radiology, pancreas, ablation, irreversible electroporation
Reversible electroporation has been studied for the treatment of cancer in combination with cytotoxic drugs. This is termed “electrochemotherapy.” Irreversible electroporation (IRE), however, is a relatively recent method of ablation, first introduced by Davalos et al. 1 In contrast to many ablation devices which use thermal methods to induce cell death, IRE employs the use of an electric field to cause irreversible permeability of the cell membrane, thus inducing apoptosis.
The use of IRE in the pancreas was first described by Martin et al 2 in a pilot study published in 2012, where the safety and efficacy of IRE in the pancreas were first explored. Since it was first described, IRE has become established as part of the armamentarium of ablation devices currently available.
While thermal ablation has been attempted in locally advanced pancreatic cancer (LAPC), this has been associated with high morbidity and even mortality. 3 The crucial advantage of IRE compared with other devices employing thermal ablation is the safety around vital structures such as vessels and ducts. This is especially important in the pancreas due to the close proximity of multiple major vascular structures, biliary ducts, and adjacent gastrointestinal organs. This article will explore the current evidence regarding the use of IRE in the pancreas.
Physics/Mechanism of Irreversible Electroporation
IRE employs the use of two or more electrodes inserted around a tumor to generate an electric field. Multiple cycles of short, high-voltage electrical pulses generated across the ablation zone alter the transmembrane potential of tumor cells. This creates defects in the lipid bilayer of the cell membrane, increasing membrane permeability. With the application of the appropriate electrical currents, this change of membrane permeability becomes permanent, leading to loss of homeostasis and thus apoptosis and cell death.
Parameters Used in Locally Advanced Pancreatic Cancer
The vast majority of centers use a setting of 90 pulses per treatment cycle, and most use a pulse length of between 70 and 90 µs. The commonly used endpoint was the completion of 90 treatment pulses. Martin et al reported the typical settings for ablation of LAPC. 4 These are summarized in Table 1 .
Table 1. Typical settings for IRE in locally advanced pancreatic cancer 4 .
| Probe spacing range typically used | 1.5–2.0 cm |
| Absolute minimum probe spacing typically used | 1.0 cm |
| Absolute maximum probe spacing typically used | 2.6 cm |
| Default system pulse length for pulse delivery | 90 µs |
| Absolute minimum pulse length typically used | 70 µs |
| Default number of pulses for pulse delivery | 70 pulses |
| Absolute minimum number of pulses typically delivered for each probe pair during pulse delivery | 70 pulses |
| Number of pulses typically delivered between each probe pair during one round of pulse delivery | 90–100 pulses |
| Maximum number of pulses typically delivered for each probe pair after pulse delivery before pull back | 180–270 pulses |
| Default voltage setting | 1,500 V/cm |
| Voltage setting range typically used | 1,400–2,000 V/cm |
| Default maximum voltage output of system | 3,000 V |
| Initial probe exposure typically used for soft tissue (i.e., liver, kidney, lung) | 2.0 cm |
| Initial probe exposure typically used for highly conductive soft tissue (i.e., pancreas) | 1.5 cm |
| Maximum probe exposure typically used for soft tissue | 2.5 cm |
| Pulse timing setting typically used for lesions outside abdominal or thoracic cavities | 90 ppm |
| Pulse timing setting typically reserved for operational verification testing | 240 ppm |
| Default voltage used for test pulse sequence | 400 V |
| Default number of pulses delivered for each probe pair for “test pulse sequence” | 1 pulse |
| Number of pulses typically delivered across each probe pair when performing “tissue conductivity test” | 20 pulses |
| Current range typically displayed after performing a “tissue conductivity test” | 20–35 A |
| Default maximum current limit of NanoKnife | 50 A |
Abbreviations: A, amperes; IRE, irreversible electroporation; ppm, pulses per minute.
Anesthetic Management
Anesthetic management during IRE differs from standard general anesthesia. The application of pulsatile high voltages carries a risk of cardiac arrhythmias and causes severe muscular contractions. There may also be a small risk of seizures due to stimulation of nervous tissue. Despite a deeper neuromuscular blockade, local contractions may still be observed as a pulsatile movement of the probes. This is worse with probes traversing large muscle groups and may be a consideration during probe placement to avoid inadvertent displacement during the procedure.
Ventricular tachycardia has been observed in IRE without the use of cardiac synchronization, 5 some of which resulted in a decrease in blood pressure. IRE should thus be performed with cardiac synchronization to the IRE pulse delivery during the absolute refractory period of the cardiac cycle. 6 With synchronized pulsing, only minor arrhythmias have been reported, all of which resolved spontaneously on completion of the treatment cycle.
An increase in systolic and diastolic blood pressure has also been reported in IRE, with the largest increases seen in ablation of the pancreas. Nielsen et al reported median increases of 60 mm Hg systolic and 30 mm Hg diastolic pressures. 6 Heart rate also increases, the extent of which is again noted to be highest in the ablations of the pancreas, with a median of 18 beats per minute. 6
These challenges, when well managed, generally allow for a safe procedure.
Locally Advanced Pancreatic Cancers
Pancreatic adenocarcinoma is the 14th most common cancer in the world and the 7th leading cause of cancer mortality. 7 It has notoriously poor survival rates, with only up to 20% of cancers deemed resectable at the time of diagnosis. Overall 5-year survival rates are less than 5%, with largely no improvement for the past few decades. Those who present with metastatic disease or locally advanced pancreatic malignancy have median survivals of between 6 and 16 months. A tumor is generally considered unresectable when there is the presence of metastasis, peripancreatic lymphadenopathy; encasement of the superior mesenteric vein or portal vein confluence; or involvement of the superior mesenteric artery, celiac axis, inferior vena cava, or aorta. Over 90% of patients with these CT findings would be deemed unresectable at operation. LAPC has been traditionally treated with chemotherapy with or without radiation. Resection may be considered if the cancer could be down staged. However, overall survival generally remains poor. Thus, there is an impetus to develop modalities to target this subgroup of patients.
Local ablative therapies for other solid organs have been developed over the past decade with varying success, in some cases even substituting surgery. However, most are thermal-based technologies like radiofrequency, microwave, and cryotherapy. The pancreas, however, presents a challenging environment for these modalities because of the close proximity of major vessels and biliary ducts. Thermal-based technologies create indiscriminate soft tissue damage through coagulative necrosis, thus limiting their use in the pancreas. IRE is currently the only ablative treatment with a nonthermal method of action and few studies have demonstrated its safety and efficacy in the pancreas. 8
Martin et al published promising results in the treatment of 200 LAPCs with IRE. The median overall survival for all patients with LAPC who underwent IRE was 23.2 months. 9 According to the data in the AHPBA registry, median overall survival was 30.7 months from diagnosis, with a mean progression-free survival of 22.8 months and a median time to progression of 27.3 months. 10 These data suggest that IRE does confer a survival advantage and should be included in the multimodality approach to pancreatic cancer.
Borderline Resectable Pancreatic Cancer
Radical surgery is achievable only in approximately 20% of patients with pancreatic cancer at diagnosis. Even after a presumed margin-negative resection, the majority of patients (50–80%) still develop local tumor recurrence. Coupled with poor 5-year survival rates of around 20%, this suggests the presence of microscopic metastases despite a presumed margin-negative resection. 11 Still, radical surgery offers the best chance of cure.
A significant number of patients have borderline resectable pancreatic cancer, usually defined as less than 180-degree encasement of the superior mesenteric artery and infiltration of the mesenteric-portal axis with a possibility of reconstruction. Induction chemotherapy will usually be employed in this group of patients with the aim of an eventual margin-negative resection (R0). More often than not, these are cases where the primary tumor lies in close proximity to major vessels or ducts, necessitating close resection margins and risking incomplete tumor resection. In these cases, various methods have been employed to attempt to achieve negative margins. These include radiotherapy as well as IRE. 11
IRE is used to augment surgical resection in a technique termed “marginal accentuation.” In this technique, IRE is performed prior to surgical resection of the threatened margin, usually after the pancreatic neck has been transected. This is mandatory as IRE requires soft tissue for placement of needles. The aim of this therapy is to achieve an electroporation zone along the threatened resection margin where microscopic disease might exist. This is common along the superior mesenteric artery/retroperitoneal margin or the base of the celiac-aortic margin. Probes are always placed under direct ultrasound guidance, usually in a caudal cephalad orientation relative to the pancreas. Several pull back ablations or repositions may be necessary to achieve an adequate ablation zone. This is usually determined by monitoring the zone with ultrasound and an assessment of the change in resistance of the ablation zone. See Figs. 1 2 3 to 4 for examples of advanced techniques used during IRE of the pancreas.
Fig. 1.
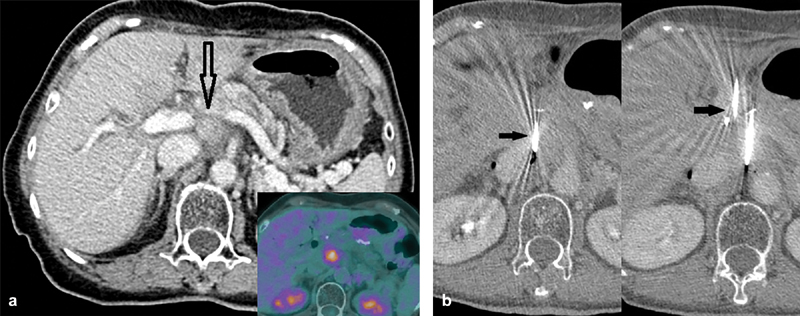
Case 1. A 73-year-old woman with recurrence of pancreatic adenocarcinoma post–pancreatico-duodenectomy (Whipple's). ( a ) FDG avid enhancing soft tissue in the surgical bed (inset). Notice the close proximity to the portal vein, inferior vena cava, celiac trunk, and splenic vein (empty black arrow). ( b ) First and second needle placement adjacent to the inferior vena cava and the aorta. Notice the needles placed parallel and ∼1.5 cm apart (arrows). Optimal spacing between IRE probes are 1.5–2.2 cm. Parallel placement is needed to ensure nondistortion of the electrical field, although in practice up to 10 degrees divergence is acceptable.
Fig. 2.
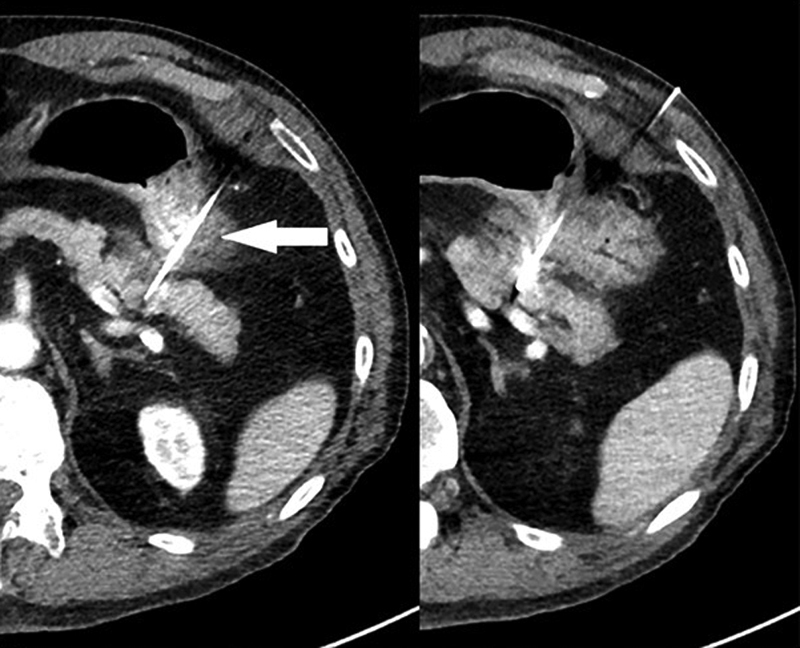
Transgastric placement of IRE probes to target an enhancing pancreatic tail lesion in a pancreatic tail lesion in a nonsurgical patient. Again, notice the parallel placement of probes, bracketing the lesion. Transgastric needle placement (arrow) was necessary due to lack of an available window. This is generally safe with the 19G IRE probes. Unlike other ablative modalities with larger probe sizes, transversal of stomach and small bowel is deemed safe.
Fig. 3.
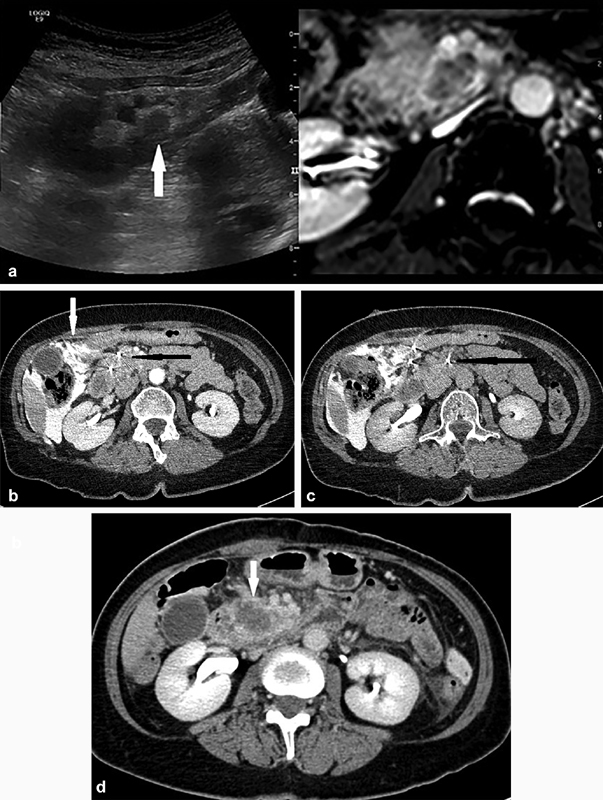
A 65-year old woman with neuroendocrine tumor in the pancreatic head and liver metastasis. The liver metastasis was successfully ablated using thermal ablation techniques with no evidence of short-term recurrence and improvement in symptoms. Decision for IRE was made at a multidisciplinary meeting for local disease control. ( a ) Ultrasound/MRI fusion image showing the hypoechoic lesion in the head of the pancreas (arrow), closely related to the superior mesenteric artery and vein anteriorly, as well as the inferior vena cava posteriorly. Probe placement under US/MRI fusion guidance in the caudal-cranial direction with position checked on CT. ( b ) CT image showing parallel needle placement (solid black arrow). Note the aggressive hydrodissection performed to create a safer window for placement of needles (white arrow). ( c ) To achieve adequate margins, a third probe was placed on the left of the superior mesenteric vessels, ∼2.0 cm away from the nearest ablation probe (black arrow). ( d ) Three-day postprocedure CT showing satisfactory ablation zone in the head of the pancreas (arrow) with no evidence of adjacent vessel injury.
Fig. 4.
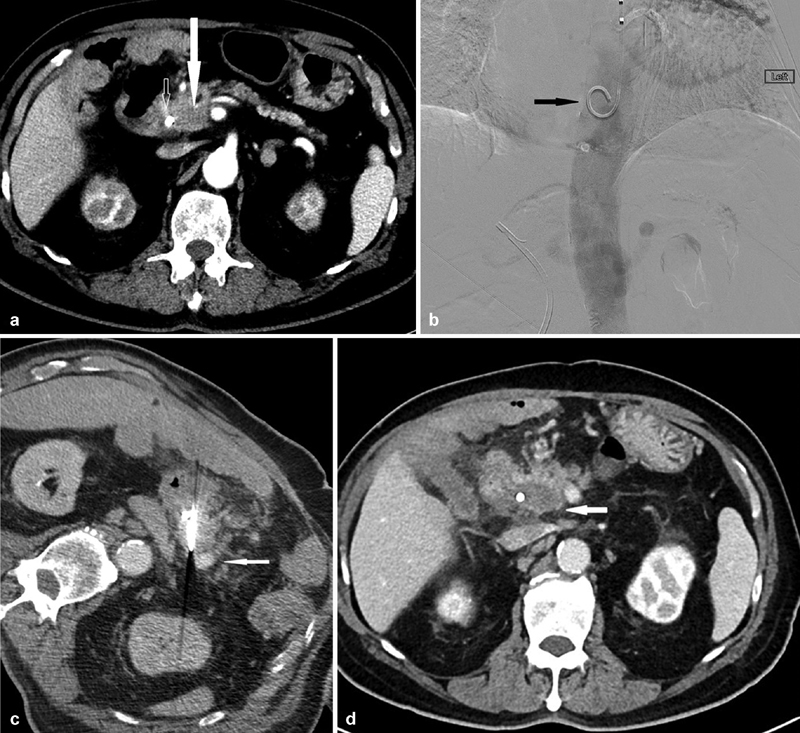
A 70-year-old man presenting with a tumor in the head of the pancreas who was medically unfit for pancreatico-duodenectomy. ( a ) Preprocedural CT showing a hypoenhancing mass (closed arrow) in the head of the pancreas surrounding by the superior mesenteric artery, hepatic artery, gastroduodenal artery, as well as the common bile duct with a biliary stent in situ (open arrow). The superior mesenteric vein has been completely obliterated by the mass. ( b ) Placement of a pigtail catheter (arrow) in the descending aorta through a left transradial approach before IRE. This aids in the visualization of the vascular anatomy through 10-mL boluses of 50% contrast during the placement of IRE probes during intermittent CT fluoroscopy. ( c ) Placement of IRE probes. Note the enhancement of the surrounding vasculature (arrow) which would otherwise be difficult to delineate without intermittent contrast boluses from the preplaced pigtailed catheter in the descending aorta. ( d ) CT 3 months postablation showing no evidence of recurrence at the ablation site (arrow) with stability in size.
Outcome of Irreversible Electroporation
A recent meta-analysis by Ansari et al 8 gathered data from 10 clinical studies for a total of 446 patients, of which 142 patients underwent percutaneous IRE while the remaining 304 had IRE during open surgery. Technical success rates for most of the smaller studies were reported to be 100%. Narayanan et al published a technical success rate of 85%, 12 though it is important to note that this was a series of 50 patients who underwent percutaneous IRE. Also, there was no standardized way of reporting technical success, with studies using postprocedure CT or ultrasound. Published recurrence-free survival rates varied between 6.1months 13 and 12.4 months. 9 Overall survival ranged between 7.0 months 13 and 23 months. 9 These studies are considerably heterogeneous and it is difficult to draw definitive conclusions from these survival rates. Other modalities of local ablation of LAPC have reported similar outcomes, with median overall survival between 5 and 25.6 months. 8 Large studies of stereotactic radiotherapy and high-intensity focused ultrasound have also reported median OS in the range of 6.2 to 24 months and 11.0 to 12.6 months. 8 A meta-analysis of patients treated with resection and chemotherapy by Gillen et al 14 published a median overall survival of 22.4 months. This is comparable than the results published by Martin et al 9 of 23.2 months and data in the AHPBA registry of 22.8 months. 10
In summary, the efficacy of IRE is yet to be firmly established. Considering the loose comparisons and heterogeneous data, larger more powerful studies are required to demonstrate its efficacy.
Complications
IRE carries a small but significant risk of mortality and morbidity. The larger studies published demonstrate a mortality rate of around 2%, 9 11 while others have quoted mortality rates from 0 to 17%. 15 16 In the meta-analyses by Ansari et al, 8 there were a total of 9 mortalities in 446 patients (2%). Severe complication rate was reported to be between 0 and 24%, 17 18 while minor complication rate ranged from 10 to 62%. 12 19
In the largest experience of 200 patients published by Martin et al, 9 20 (40%) of the resected patients and 54 (36%) of the nonresected patients experienced a total of 49 and 100 complications, respectively. Of note, many of the patients in this series were also treated with extensive surgery. Three deaths (2%) occurred in the nonresected group, due to bleeding from ulcerated tumor in the duodenum, liver failure, and pulmonary embolism. There were no deaths in the group subjected to IRE and simultaneous pancreatic resection. While the comparison of complications is difficult due to differing standards among publications, major complications have been reported to occur in 13%. 10 These included upper gastrointestinal hemorrhage, visceral perforations, pancreatic leak, clinical pancreatitis, and pancreatic failure, as well as vascular complications such as pseudoaneurysm, hepatic arterial thrombosis, and mesenteric/portal vein thrombosis. Ascites, biliary anastomotic stricture, and liver dysfunction/failure have also been reported. However, in an article by Martin et al 20 comparing 54 patients who underwent IRE propensity matched to 85 stage III patients with standard chemoradiotherapy, it was demonstrated that the morbidity of this additional IRE treatment was similar to continued systemic chemotherapy after the 4-month induction treatment. There were equally severe complications with continuing chemotherapy after the 4- to 6-month induction period when compared with the IRE group. These suggest that continued chemotherapy has similar morbidity when compared with IRE treatment delivery. The morbidity of IRE thus maybe offset by the significant improvement in overall survival and local PFS.
Post-IRE Follow-up and Imaging Appearances
In a swine model, Fritz et al reported that the post-IRE ablation zone appears as a hypodense lesion with clear demarcation, and was best visualized in the portal venous phase. After 7 days, no significant changes were seen. 21 Another porcine model published by Wimmer et al reported that hypodense ablation zones and edema were seen 15 minutes after IRE and best demarcated 20 to 40 seconds after contrast injection. There was an increase in edema after 24 hours. Similar findings were noted at 14 and 28 days, where the ablation zone was overall decreased in size and signs of edema were absent. 22
In humans, Akinwande et al reviewed five patients who underwent IRE for LAPC. Of note, this study was conducted in cases of pancreatic cancer in humans, and thus better represents what may be expected in the relevant clinical context. They reported that the arterial phase is the best for postoperative imaging to distinguish the hypoattenuating ablation zone from adjacent vasculature. They found the ablation zone to be irregular and shapeless on imaging without clear margins. 23 They also reported that vessels within the ablation zones showed narrowing immediately post-IRE but resolved or remained stable in follow-up scans. Longer follow-up imaging showed increased enhancement of the ablation zone which suggests the development of granulation tissue and fibrosis. After the resolution of surrounding edema, longer postprocedure scans showed a smaller ablation zone when compared with the initial postoperative scans. Due to the lack of defined margins, the authors concluded that size is a secondary objective on CT evaluation. However, the increase in the size of the ablation zone or any new encasement or narrowing of vessels or extension of soft tissue outside the ablation zone is concerning for recurrence.
With regard to nontumor findings, Schulz et al retrospectively investigated nontumor findings on CT within 30 days of IRE 24 in 36 patients. Most of these were seen within the first 2 weeks post-IRE. Most common were vascular complications, with narrowing/compression or occlusion of a major vessel seen almost 2 weeks after IRE. Nonspecific bowel wall edema was the most common gastrointestinal tract complication. Bowel perforation, portal venous gas, gastrointestinal hemorrhage, and pneumatosis intestinalis were seen less frequently. Biliary tree abnormalities, such as common bile duct dilatation and pancreatic ductal gas, were less common and typically delayed. A separate study by Gonzalez-Beicos et al found no evidence of vascular narrowing or obliteration, 25 though it is noteworthy that this study included only three patients who underwent IRE in the pancreas.
While there is no consensus on when follow-up should be performed, Martin et al 4 recommended an immediate triple-phased CT scan in the plain, arterial, and venous phased within 1 month to access patency of vital structures. This is followed by serial imaging for 2 to 6 months to detect recurrence. A proposed radiologic criterion for pancreatic IRE assessment is represented in Table 2 .
Table 2. Radiologic criteria for pancreatic IRE assessment 4 .
| CR | PR | SD | PD | |
|---|---|---|---|---|
| Major criteria (1 sufficient for PD) | ||||
| Longest diameter in the axial plane of the soft-tissue component of primary tumor compared with the 1st follow-up scan >3 mo after treatment Metastases (non-nodal) with longest diameter >10 mm |
No residual soft-tissue primary tumor | Decrease >30% | Decrease <30% to increase <20% | Increase >20% |
| No | No | No | Yes | |
| Minor criteria (>2 needed for PD in absence of major criteria) | ||||
| Vessel narrowing (compared with the 1st follow-up scan >3 mo after treatment; >50% diameter reduction; no thrombosis. Biliary stents or endoprosthesis) | No | Yes | ||
| New-onset biliary obstruction (compared with the 1st follow-up scan >3 mo after treatment; no biliodigestive anastomosis, biliary stents or endoprosthesis) | No | Yes | ||
| Enlarging area of diffusion restriction on MRI (compared with the 1st follow-up MRI > 3 mo after treatment) | No | Yes | ||
| New lymph nodes >15 mm short-axis diameter or pathology proven (compared with the 1st follow-up scan > 3 mo after treatment) | No | Yes | ||
| CA 19.9 increase >100% (and >74 U/mL) without signs for pancreatitis or biliary duct obstruction (compared with pretreatment values) | No | Yes | ||
Abbreviations: CR, complete response; IRE, irreversible electroporation; MRI, magnetic resonance imaging; PD, progressive disease; PR, partial response; SD, stable disease.
Troubleshooting and Technical Considerations
Tissue Conductivity Test
Initial energy delivery should start at a probe exposure of 1 to 1.5 cm, 1,500 V/cm with a pulse length of 90 ms per probe pair. The probes should be evenly spaced with no convergence or divergence at the tip. Probe positions should be checked every time the probes are readjusted/pulled back to ensure that they are parallel. 4
After probes are placed in position, a tissue conductivity test is performed using 20 pulses per pair of electrodes. The test results will have to be reviewed to ensure that the starting amperage level is within a range of 20 to 35A for each probe pair.
If the amperage level is out of this range, the probes should be readjusted and the tissue conductivity test repeated. If the amperage is too low, this may be due to wide probe spacing (>2.5 cm). If probe spacing is less than 2 cm but the amperage is still low, volts per centimeter may be increased by 200 to 400 V/cm for the probes involved. If the amperage is still low, the probe exposure may be increased by 0.5 to 1.0 cm. If the amperage is too high, ensure that the probes are not placed too close a proximity (<1.0 cm). Probe exposure may be decreased to a minimum of 1.0 cm. The pulse length may also be reduced from 90 to 80 or 70 mm, especially if there is a sharp rise in amperage across each pulse. If amperage is still high, the volts per centimeter may be reduced by 200 to 400 V/cm for the affected probe pairs. This, however, will lead to a decrease in the size of the ablation zone.
Once the tissue conductivity test is successful with the desired amperage within 20 to 35 A, the “baseline current” should be noted and referenced for the procedure. The number of pulses should be changed to 90 to 100 and the ablation commenced. At the end of the ablation cycle, there should be an increase of at least 12 A from the baseline current for each probe pair. If the increase in current is insufficient, an additional 90 pulses should be delivered for the probe pair in question. If the last few probe pairs do not achieve a 12-A rise in current, this is probably due to the fact that the tissue has already been ablated. Commonly, not more than 270 pulses are administered for a probe pair in the same ablation zone, as it is believed to not carry any benefit.
After completion of the procedure, the probes may be removed.
Future Applications of IRE in the Pancreas
Tarantino et al reported the use of IRE in a locally advanced solid pseudopapillary carcinoma (SPC) of the pancreas. 26 The patient was a 24-year old female with an aggressive large SPC of the pancreas with locally aggressive behavior involving the splenic vein and celiac artery. The patient was treated with six insertions of three to four electrodes per insertion. Postprocedure up to 48 months showed no local or distal recurrence. The authors concluded that in cases with a poor indication for surgery, IRE could be a safe and effective alternative therapy for advanced staged pancreatic SPC, and could be a bridge treatment to radical surgery.
Niu et al also reported the use of IRE in the treatment of a locally advanced VIPoma. 27 Postablation, there was a large area of necrosis in the mass with a subsequent decrease in symptoms and normalization of VIP hormone levels. Similarly, IRE has been used in the ablation of an insulinoma. 28
Other novel methods of probe placement have been explored. Lee et al reported the use of endoscopic ultrasound–guided pancreatic IRE in the porcine pancreas. 29 The gastrointestinal route may be a promising approach to pancreatic lesions given its proximity. Tartaglia et al 30 reported the use of IRE for LAPC through a laparoscopic approach in a single patient with needle placement using laparoscopic ultrasound.
Conclusion
The use of IRE in the treatment of LAPC is still in its early stages. While the modality does have an acceptable risk profile, further studies regarding its efficacy is required. Nevertheless, it is a promising and important new tool in the treatment of LAPC and may confer benefit in a carefully selected patient population.
Footnotes
Conflict of Interest None declared.
References
- 1.Davalos R V, Mir I L, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng. 2005;33(02):223–231. doi: 10.1007/s10439-005-8981-8. [DOI] [PubMed] [Google Scholar]
- 2.Martin R C, II, McFarland K, Ellis S, Velanovich V. Irreversible electroporation therapy in the management of locally advanced pancreatic adenocarcinoma. J Am Coll Surg. 2012;215(03):361–369. doi: 10.1016/j.jamcollsurg.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 3.D'Onofrio M, Barbi E, Girelli R et al. Radiofrequency ablation of locally advanced pancreatic adenocarcinoma: an overview. World J Gastroenterol. 2010;16(28):3478–3483. doi: 10.3748/wjg.v16.i28.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin R C, II, Durham A N, Besselink M G et al. Irreversible electroporation in locally advanced pancreatic cancer: A call for standardization of energy delivery. J Surg Oncol. 2016;114(07):865–871. doi: 10.1002/jso.24404. [DOI] [PubMed] [Google Scholar]
- 5.Ball C, Thomson K R, Kavnoudias H. Irreversible electroporation: a new challenge in “out of operating theater” anesthesia. Anesth Analg. 2010;110(05):1305–1309. doi: 10.1213/ANE.0b013e3181d27b30. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen K, Scheffer H J, Vieveen J M et al. Anaesthetic management during open and percutaneous irreversible electroporation. Br J Anaesth. 2014;113(06):985–992. doi: 10.1093/bja/aeu256. [DOI] [PubMed] [Google Scholar]
- 7.Bray F, Ferlay J, Soerjomataram I, Siegel R L, Torre L A, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(06):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 8.Ansari D, Kristoffersson S, Andersson R, Bergenfeldt M. The role of irreversible electroporation (IRE) for locally advanced pancreatic cancer: a systematic review of safety and efficacy. Scand J Gastroenterol. 2017;52(11):1165–1171. doi: 10.1080/00365521.2017.1346705. [DOI] [PubMed] [Google Scholar]
- 9.Martin R C, II, Kwon D, Chalikonda Set al. Treatment of 200 locally advanced (stage III) pancreatic adenocarcinoma patients with irreversible electroporation: safety and efficacy Ann Surg 201526203486–494., discussion 492–494 [DOI] [PubMed] [Google Scholar]
- 10.Holland M M, Bhutiani N, Kruse E Jet al. A prospective, multi-institution assessment of irreversible electroporation for treatment of locally advanced pancreatic adenocarcinoma: initial outcomes from the AHPBA pancreatic registryHPB (Oxford)2019. doi: 10.1016/j.hpb.2018.12.004. [Epub ahead of print] [DOI] [PubMed]
- 11.Kwon D, McFarland K, Velanovich V, Martin R C., II Borderline and locally advanced pancreatic adenocarcinoma margin accentuation with intraoperative irreversible electroporation. Surgery. 2014;156(04):910–920. doi: 10.1016/j.surg.2014.06.058. [DOI] [PubMed] [Google Scholar]
- 12.Narayanan G, Hosein P J, Beulaygue I C et al. Percutaneous image-guided irreversible electroporation for the treatment of unresectable, locally advanced pancreatic adenocarcinoma. J Vasc Interv Radiol. 2017;28(03):342–348. doi: 10.1016/j.jvir.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 13.Månsson C, Brahmstaedt R, Nilsson A, Nygren P, Karlson B M. Percutaneous irreversible electroporation for treatment of locally advanced pancreatic cancer following chemotherapy or radiochemotherapy. Eur J Surg Oncol. 2016;42(09):1401–1406. doi: 10.1016/j.ejso.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 14.Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7(04):e1000267. doi: 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narayanan G, Hosein P J, Arora G et al. Percutaneous irreversible electroporation for downstaging and control of unresectable pancreatic adenocarcinoma. J Vasc Interv Radiol. 2012;23(12):1613–1621. doi: 10.1016/j.jvir.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Kluger M D, Epelboym I, Schrope B A et al. Single-institution experience with irreversible electroporation for T4 pancreatic cancer: first 50 patients. Ann Surg Oncol. 2016;23(05):1736–1743. doi: 10.1245/s10434-015-5034-x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Shi J, Zeng J et al. Percutaneous irreversible electroporation for ablation of locally advanced pancreatic cancer: experience from a Chinese institution. Pancreas. 2017;46(02):e12–e14. doi: 10.1097/MPA.0000000000000703. [DOI] [PubMed] [Google Scholar]
- 18.Lambert L, Horejs J, Krska Z et al. Treatment of locally advanced pancreatic cancer by percutaneous and intraoperative irreversible electroporation: general hospital cancer center experience. Neoplasma. 2016;63(02):269–273. doi: 10.4149/213_150611N326. [DOI] [PubMed] [Google Scholar]
- 19.Belfiore M P, Ronza F M, Romano F et al. Percutaneous CT-guided irreversible electroporation followed by chemotherapy as a novel neoadjuvant protocol in locally advanced pancreatic cancer: our preliminary experience. Int J Surg. 2015;21 01:S34–S39. doi: 10.1016/j.ijsu.2015.06.049. [DOI] [PubMed] [Google Scholar]
- 20.Martin R C, II, McFarland K, Ellis S, Velanovich V. Irreversible electroporation in locally advanced pancreatic cancer: potential improved overall survival. Ann Surg Oncol. 2013;20 03:S443–S449. doi: 10.1245/s10434-012-2736-1. [DOI] [PubMed] [Google Scholar]
- 21.Fritz S, Sommer C M, Vollherbst D et al. Irreversible electroporation of the pancreas is feasible and safe in a porcine survival model. Pancreas. 2015;44(05):791–798. doi: 10.1097/MPA.0000000000000331. [DOI] [PubMed] [Google Scholar]
- 22.Wimmer T, Srimathveeravalli G, Gutta N et al. Comparison of simulation-based treatment planning with imaging and pathology outcomes for percutaneous CT-guided irreversible electroporation of the porcine pancreas: a pilot study. J Vasc Interv Radiol. 2013;24(11):1709–1718. doi: 10.1016/j.jvir.2013.05.056. [DOI] [PubMed] [Google Scholar]
- 23.Akinwande O, Ahmad S S, Van Meter T, Schulz B, Martin R C. CT findings of patients treated with irreversible electroporation for locally advanced pancreatic cancer. J Oncol. 2015;2015:680319. doi: 10.1155/2015/680319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulz B, Ou J, Van Meter T, Martin R C. Early nontumorous CT findings after irreversible electroporation of locally advanced pancreatic cancer. Abdom Radiol (NY) 2016;41(11):2142–2149. doi: 10.1007/s00261-016-0815-7. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Beicos A, Venkat S, Songrug T et al. Irreversible electroporation of hepatic and pancreatic malignancies: radiologic-pathologic correlation. Tech Vasc Interv Radiol. 2015;18(03):176–182. doi: 10.1053/j.tvir.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Tarantino L, Nasto A, Busto G, Iovino V, Fristachi R, Bortone S. Irreversible electroporation of locally advanced solid pseudopapillary carcinoma of the pancreas: a case report. Ann Med Surg (Lond) 2018;28(28):11–15. doi: 10.1016/j.amsu.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niu L, Li J, Zeng J et al. Percutaneous irreversible electroporation for pancreatic VIPoma: a case report. Pancreas. 2017;46(01):135–137. doi: 10.1097/MPA.0000000000000698. [DOI] [PubMed] [Google Scholar]
- 28.Mele C, Brunani A, Damascelli B et al. Non-surgical ablative therapies for inoperable benign insulinoma. J Endocrinol Invest. 2018;41(02):153–162. doi: 10.1007/s40618-017-0738-3. [DOI] [PubMed] [Google Scholar]
- 29.Lee J M, Choi H S, Chun H J et al. EUS-guided irreversible electroporation using endoscopic needle-electrode in porcine pancreas. Surg Endosc. 2019;33(02):658–662. doi: 10.1007/s00464-018-6425-4. [DOI] [PubMed] [Google Scholar]
- 30.Tartaglia E, Fabozzi M, Rizzuto A et al. Irreversible electroporation for locally advanced pancreatic cancer through a minimally invasive surgery supported by laparoscopic ultrasound. Int J Surg Case Rep. 2018;42:290–294. doi: 10.1016/j.ijscr.2017.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]


