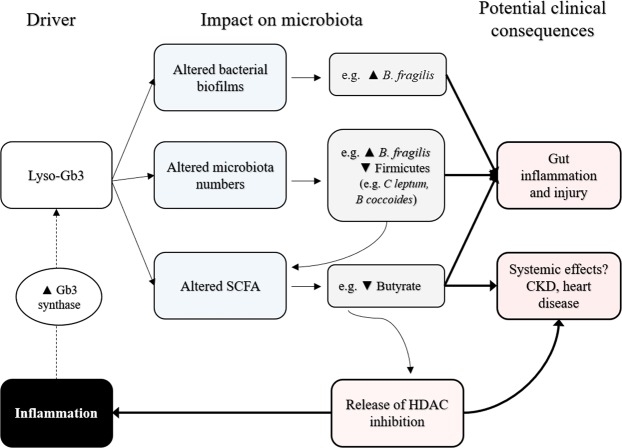
Figure 4.
Summary of research findings and working hypothesis for possible clinical implications. At clinically relevant concentrations, lyso-Gb3 modified the biofilm-forming properties and counts of diverse bacteria. Most notably, lyso-Gb3 consistently increased B. fragilis biofilm formation or counts in three different, independent experimental settings. Additionally, it led to decreased production of butyrate and an altered pattern of other short-chain fatty acids (SCFA). Experimental data from the literature have linked low butyrate levels to gastrointestinal manifestations and kidney and heart disease, which are also found in Fabry disease. Thus, butyrate has been reported to have anti-inflammatory properties attributable to its ability to inhibit histone deacetylases (HDACs)23,79–81. In this regard, we hypothesize that lyso-Gb3-induced lowering of butyrate levels may release the inhibition of butyrate on HDACs and favor systemic inflammation. Inflammation, in turn, is known to increase the activity of Gb3 synthase77, thus increasing the synthesis of metabolites such as Gb3 that accumulate in Fabry disease and potentially increasing the severity of kidney and heart disease.