Abstract
The biotransformation and detoxification mechanisms of arsenic (As) species have been active research topics because of their significance to environmental and human health. Biotransformation of As in phytoplankton has been extensively studied. However, how different growth phases of phytoplankton impact As biotransformation in them remains uncertain. This study investigated the biotransformation of As species in freshwater phytoplankton at different growth phases to ascertain at which growth phase different types of biotransformation occur. At the logarithmic growth phase, arsenate (AsV) (>90%) and arsenite (AsIII) (>80%) predominated in culture media when phytoplankton were exposed to 20 nmol L−1 and 1.0 µmol L−1 of AsV, respectively, and methylarsenic (methylAs) species were not detected in them at all. Intracellular As was mainly present in inorganic forms (iAs) at the logarithmic phase, while substantial amounts of organoarsenic (orgAs) species were detected at the stationary phase. At the stationary phase, AsV comprised the majority of the total As in culture media, followed by AsIII and methylAs, although the methylation of AsV occurred slowly at the stationary phase. Biotransformation of AsV into AsIII and As methylation inside phytoplankton cells occurred mainly at the logarithmic phase, while the biotransformation of As into complex orgAs compounds occurred at the stationary phase. Phytoplankton rapidly released iAs and methylAs species out of their cells at the logarithmic phase, while orgAs mostly remained inside their cells.
Subject terms: Environmental impact, Freshwater ecology
Introduction
Arsenic (As), an environmental pollutant, is extremely toxic to living organisms at high concentrations. The occurrence of As in aquatic systems is of great concern due to its high bioavailability, bioaccumulation, and trophic transfer from the bases of aquatic food chains through to higher trophic levels1. Arsenic in marine biota may not be a significant concern for human health because it is present among them in low concentrations. However, As in freshwater systems is likely to be a significant environmental and human health problem due to the high concentrations that can result from its direct input into these systems from natural and manmade sources2. Arsenic exists in different chemical forms in aquatic systems. Two major As species in aquatic systems are arsenate (AsV), which is the most thermodynamically stable form in oxic waters, and arsenite (AsIII), which is predominant in reduced-oxygen environments3,4. Through biotransformation processes, microorganisms like phytoplankton and bacteria can cause significant changes in the biogeochemistry of As in aquatic systems5–8. Photosynthetic microorganisms (e.g., phytoplankton and cyanobacteria) are able to accumulate AsV and biotransform it into AsIII and methylarsenic (methylAs) species, such as monomethylarsonate (MMAA) and dimethylarsinate (DMAA)9. Although the toxicity of AsIII is higher than that of AsV, AsIII is predominantly excreted from cells, whereas AsV is excreted less. Therefore, a number of researchers have suspected that the reduction of AsV to AsIII represents a detoxification mechanism of phytoplankton2.
Arsenic biotransformation by microorganisms plays a significant role in the occurrence, toxicity, and biogeochemistry of this toxic element in the aquatic environment. Several pathways of As biotransformation have been proposed in different microorganisms that are mainly related to oxidation or reduction reactions10. The microorganisms conduct these redox reactions either to protect themselves from the toxic effects of this metalloid (as a detoxification mechanism)11,12 or to produce energy to promote cellular growth10,13. Due to the physicochemical similarities between AsV (AsO43−) and phosphate (PO43−), phytoplankton actively take up AsV through the PO43− uptake system, and then biotransform AsV inside their cells14–16. The high toxicity of AsV results because it binds to PO43− receptors that have essential functions inside cells17. To reduce its toxicity, phytoplankton biotransform AsV inside their cells in a process that involves the two-electron reduction of AsV to AsIII, which is mediated by glutathione18. The biotransformation of AsV into AsIII and its subsequent methylation to form methylAs species in phytoplankton has been reported in many previous studies19–23. Several studies also showed that the rates of AsV reduction and methylation in freshwater environments are generally dependent on the occurrence of phytoplankton blooms, which is related to nutrient enrichment and seasonal variables, such as light and temperature3,6,24. Sohrin et al.25 reported an increase in levels of AsIII in the water during the spring, which was correlated with the growth phases of two distinct phytoplankton blooms. They also observed that PO43¯ and AsV were also rapidly taken up by phytoplankton cells during these blooms. However, at the stationary bloom phase, when growth is limited by limited nutrient availability, the rates of As uptake and metabolism in phytoplankton were slow, which allowed for the further biotransformation of the pentavalent AsV into the trivalent AsIII and its subsequent methylation to DMAA to occur17. Although Sohrin et al.25 identified a relationship between the growth phases of phytoplankton blooms and the uptake and metabolism of AsV, little is known about the impacts of different phytoplankton growth phases on As biotransformation inside their cells, as well as the excretion of As metabolites out of their cells. The present study was carried out to address this knowledge gap and reveal the role of phytoplankton in As biogeochemistry in aquatic environments. The diversity in the biotransformation of and behavioral responses to As species by phytoplankton at different growth phases were also reported in this study.
Results and Discussion
Growth inhibition effects of arsenic species on freshwater phytoplankton
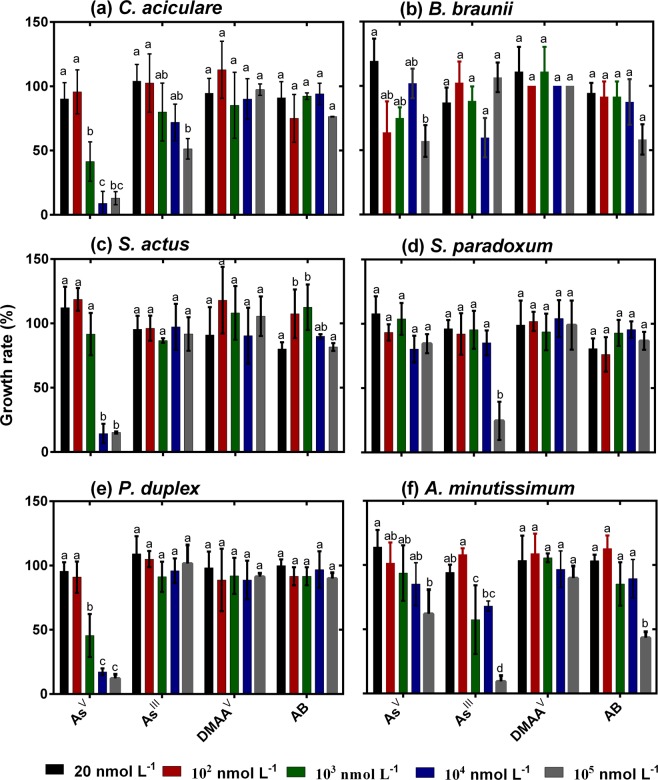
Inorganic As species had significant growth inhibition effects on freshwater phytoplankton (Fig. 1). In general, the growth of phytoplankton was inhibited substantially by high AsV concentrations (≥103 nmol L−1), except for S. paradoxum. The growth rates of C. aciculare, S. actus, and P. duplex were decreased by over 80% in the 104 nmol L−1 arsenate treatment (Fig. 1a,c,e). In contrast, the growth inhibition effects of AsV on B. braunii, S. paradoxum, and A. minutissimum at a high concentration (105 nmol L−1) were less than its effects on C. aciculare, S. actus, and P. duplex (Fig. 1b,d,f). The growth inhibition effect of AsIII on freshwater phytoplankton was lower than that of AsV (Fig. 1). The toxicity of AsIII reduced the growth rates of C. aciculare, S. paradoxum, and A. minutissimum by between 66 and 80% at an AsIII concentration of 105 nmol L−1 (Fig. 1a,d,f). The freshwater phytoplankton S. actus and P. duplex were quite resistant to AsIII toxicity, even at high concentrations (Fig. 1c,e). These results indicate that AsV and AsIII have different levels of toxicity to different types of freshwater phytoplankton.
Figure 1.
Growth of freshwater phytoplankton under various [As]0 treatments. The different lowercase letters indicate significant differences between arsenic treatments (p < 0.05). The data presented are mean ± SD growth rates (n = 3).
Methyl- and organoarsenicals, such as DMAAV and arsenobetaine (AB), respectively, had little growth inhibition effects on the tested freshwater phytoplankton (Fig. 1). These results suggest that the relative toxicity of different As species to freshwater phytoplankton decreased in the following order: AsV ≥ AsIII ≥ DMAAV ≥ AB. This also suggests that the growth inhibition of phytoplankton by As species depends on the phytoplankton strain, which is consistent with the results of other studies11,26–28. Some strains of freshwater phytoplankton are more resistant to AsV and AsIII toxicity, while others are more susceptible. This might be due to them having differential abilities to detoxify and/or biotransform AsV and AsIII into less toxic organoarsenic compounds2,26.
Phytoplankton reduce AsV to AsIII, which is then followed by the oxidative methylation of this As species to form intermediate trivalent methylAs species (MMAAIII and DMAAIII) and pentavalent methylAs species (MMAAV and DMAAV) in them18. A study by Hasegawa et al.14 reported that the freshwater phytoplankton C. aciculare converted approximately 80% of AsV into the less toxic DMAAV, with trace concentrations of trivalent methylAs species (MMAAIII and DMAAIII) also formed. The biotransformation of pentavalent AsV by freshwater phytoplankton has been discussed in detail elsewhere6.
Based on the toxic effects (i.e. growth inhibition) of iAs species on them at high concentrations (≥104 nmol L−1), the freshwater phytoplankton tested herein can be grouped into the following three distinct groups: (i) B. braunii, which is highly resistant to iAs and organoarsenic (orgAs) species; (ii) S. actus and P. duplex, which are highly resistant to AsIII and orgAs species, as well as S. paradoxum, which is highly resistant to AsV and orgAs species; and (iii) A. minutissium and C. aciculare which are highly susceptible to iAs species (AsV and AsIII).
Biotransformation of iAs species at different growth phases
Biotransformation of AsV into AsIII
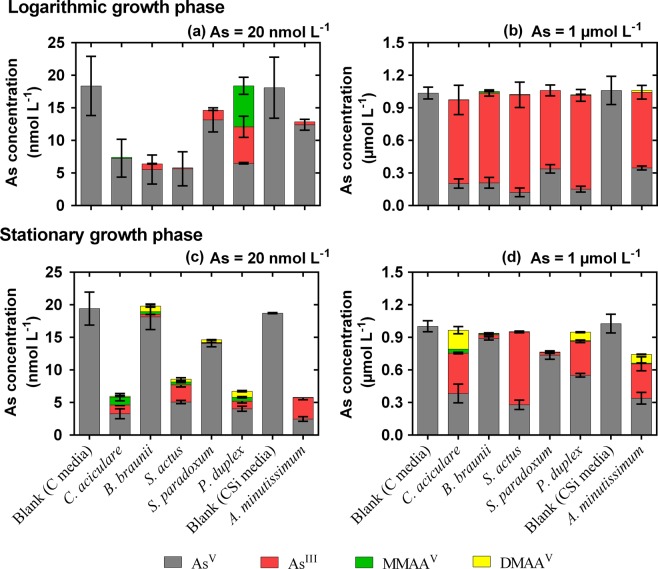
The changes in As speciation in the phytoplankton culture media at the logarithmic and stationary growth phases at high and low As concentrations are shown in Fig. 2. These results reflect the bioaccumulation and biotransformation of As species by the phytoplankton inside their cells and their subsequent excretion into the environment. In general, in the low-[As]0 treatment (20 nmol L−1), AsV was the predominant species in the growth medium, followed by AsIII, at both the logarithmic and stationary growth phases (Fig. 2a,c). However, in the high-[As]0 treatment (1.0 µmol L−1), AsIII was the predominant species in the medium at the logarithmic growth phase (Fig. 2b), while AsV was predominant at the stationary growth phase of all microalgae except S. actus (Fig. 2d).
Figure 2.
Arsenic speciation changes in the culture medium of freshwater phytoplankton at the logarithmic (a,b) and stationary (c,d) growth phases. Initially, the phytoplankton were grown in CSi culture medium with 20 nmol L−1 (a,c) and 1 μmol L−1 (b,d) of AsV. Mean ± SD As concentrations are shown (n = 3).
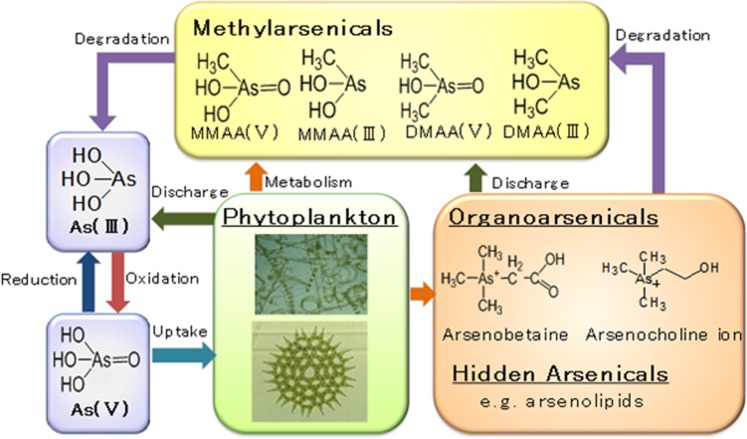
Phytoplankton plays significant roles in the biotransformation and biogeochemistry of As in aquatic systems, as illustrated in Fig. 3. Because of the physicochemical similarities between arsenate and phosphate, phytoplankton actively uptake AsV through the PO43− uptake system2,14,29. In the present study, phytoplankton were exposed to NaH2AsO4·7H2O, and the concentration ratio of arsenate to phosphate in the growth medium was kept high to encourage arsenate uptake by phytoplankton. Arsenate has toxic effect on phytoplankton (as discussed in section 2.1.) due to the binding of AsO43− to places inside the cells where PO43− binding is essential17. The phytoplankton biotransform AsV into AsIII inside their cells26,27,30, possibly to reduce the toxicity of AsV to them2. The biotransformation process involves the two-electron reduction of the pentavalent AsV to the trivalent AsIII, which is mediated by glutathione18.
Figure 3.
Biotransformation of As species by phytoplankton in aquatic systems.
The biotransformation of AsV into AsIII and its subsequent methylation to form methylarsenic compounds (methylAs; e.g., DMAA and MMAA) and more complex organoarsenic compounds (orgAs; e.g., AB) by phytoplankton are correlated with the growth rate of the phytoplankton, and to their phosphorus nutrient status17. The phytoplankton take up higher amounts of AsV than PO43−, reduce AsV to AsIII, and then excrete AsIII out of their cells as their growth rates decline and under phosphate-depleted conditions2. AsIII is further biotransformed into methylAs and more complex orgAs species, which are then excreted out of the cells. The present study further showed that the biotransformation of AsV into AsIII by freshwater phytoplankton was slow at the logarithmic growth phase in the low-[As]0 treatment. However, most of the AsIII was excreted out of the cells of the phytoplankton at this growth phase in the high-[As]0 treatment.
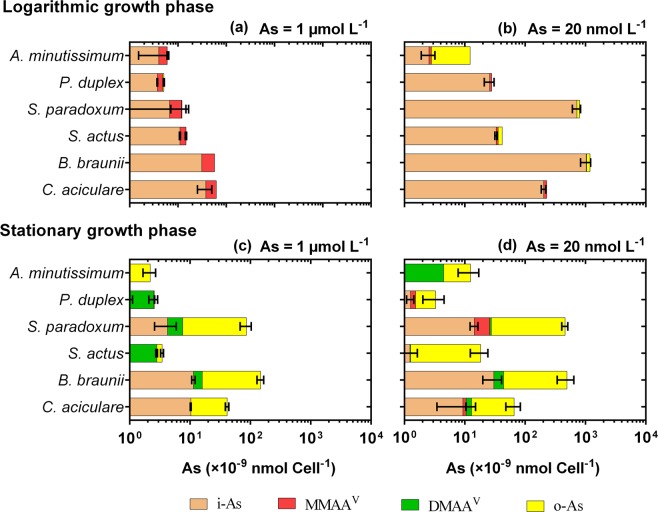
In the high-[As]0 treatment, the dominant species of As in the growth medium at the logarithmic phase was AsIII (Fig. 2b), whereas in the low-[As]0 treatment AsIII was the elast prevalent in the medium at this stage (Fig. 2a). However, the relative abundances of AsV and AsIII in the phytoplankton cells were similar in the high- and low-[As]0 treatments (Fig. 4a,b). These results demonstrated that the biotransformation of AsV into AsIII inside the cells was not affected by the concentration of [As]0 in the surrounding medium. However, the excretion of AsIII out of the cells was affected by the [As]0 in the surrounding medium. This might have been due to the fact that: (i) although AsIII is more toxic than AsV is31, AsIII is more easily excreted than AsV from the cells2; and/or (ii) the excretion of AsIII out of the cells (efflux, which many researchers have agreed occurs to reduce the toxic effects of AsIII2,8,17) is negatively correlated to the As concentration in the surrounding medium due to equilibrium effects27. The present study also showed that, unlike in the high-[As]0 treatment, AsIII excretion out of the phytoplankton cells in the low-[As]0 treatment happened at the logarithmic and stationary growth phases. However, the excretion rate of AsIII was higher at the logarithmic phase than that at the stationary phase.
Figure 4.
Arsenic speciation in freshwater phytoplankton cells at the logarithmic (a,b) and stationary (c,d) growth phases. Initially, the phytoplankton were grown in CSi culture medium with 1.0 µmol L−1 (a,c) or 20 nmol L−1 (b,d) of AsV. Mean ± SD As concentrations are shown (n = 3).
Biotransformation of iAs into methylAs and orgAs
Irrespective of the [As]0 treatment, iAs species (AsV + AsIII) were the predominant As species in the growth medium at the logarithmic growth phase (Fig. 2a,b). Intracellular As speciation results also showed that iAs species (AsV + AsIII) were the main As species inside the cells at the logarithmic growth phase, although a small amount of methylAs (mainly MMAA) was also found inside the cells at this growth phase (Fig. 4a,b). A significant proportion of the total As (t-As) inside the cells was represented by methylAs and orgAs species at the stationary growth phase (Fig. 4c,d), while the methylAs and orgAs concentrations in the growth medium were insignificant compared to the iAs concentrations therein (Fig. 2c,d). These results indicated that after the phytoplankton biotransformed iAs into methylAs and orgAs species inside their cells, these species were not excreted out of the cells by the organisms. This can be explained by the low toxicity and efflux rates of methylAs and orgAs species.
Inside the phytoplankton cells, AsV is reduced to AsIII, which is then followed by the oxidative methylation of intermediate trivalent methylAs species (MMAAIII and DMAAIII) to form pentavalent methylAs species (MMAAV and DMAAV)18. A number of freshwater phytoplankton have been reported to biomethylate iAs19,22. It is known that methylAs and orgAs species are less toxic than iAs species31. It is widely accepted that phytoplankton employ two main strategies to reduce the toxic effect of iAs: (i) the excretion of iAs (mainly AsIII) out of their cells; and (ii) the biotransformation of toxic iAs into less toxic methylAs and orgAs species2. As methylAs and orgAs species are less toxic to them, phytoplankton do not need to excrete them out of their cells, and therefore a significant amount of methylAs and orgAs were found inside the phytoplankton cells. This result also suggests that the methylation of AsV occurs more slowly than its reduction, and also differs among strains of freshwater phytoplankton.
Diversity in As biotransformation by freshwater phytoplankton
An interesting pattern in the As biotransformation performed by the six freshwater phytoplankton strains studied in the present study was observed that potentially explains the diversity of As biotransformation by phytoplankton. Based on the biotransformation efficiency of different As species, the freshwater phytoplankton examined herein could be categorized into three groups: (i) phytoplankton that are efficient at biotransforming AsIII into AsV (e.g., B. braunii and S. paradoxum); (ii) phytoplankton that cannot efficiently biotransform As, and thus rather maintain AsV inside their cells (e.g., S. actus and P. duplex); and (iii) phytoplankton that are efficient at biotransforming iAs into methylAs species (methylation) and complex orgAs species (e.g., A. minutissium, and C. aciculare) (Fig. 5; Groups A, B, and C, respectively).
Figure 5.
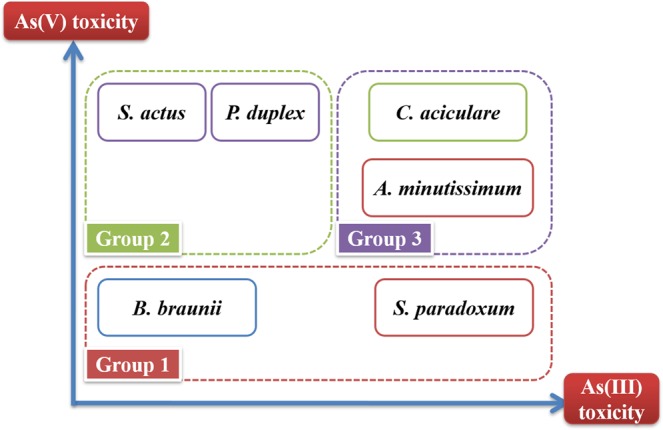
Diversity of As biotransformation by freshwater phytoplankton. All of the six-freshwater phytoplankton tested converted AsV into AsIII at the logarithmic growth phase. However, the phytoplankton could be grouped into the following three groups based on their As biotransformation ability at the stationary growth phase. Group 1: B. braunii and S. paradoxum converted AsIII into AsV; Group 2: S. actus and P. duplex maintained AsV in their cells; and Group 3: A. minutissium and C. aciculare converted iAs into methylarsenic species.
Although there is no evidence to explain why different phytoplankton species have different As biotransformation efficiencies, this is likely related to the differential physiological activities of the phytoplankton, such as bioaccumulation, detoxification (transformation of toxic iAs species into less toxic methylAs and orgAs species), and excretion of As out of the cells. The fact that B. braunii and S. paradoxum (Group A phytoplankton; Fig. 5) convert AsIII to AsV indicates that, once they are released into the oxic medium, then AsIII, MMAA, and DMAA are transformed back into the thermodynamically static AsV. In terms of AsIII biotransformation, the bio-oxidation process plays a vital role in freshwater algal cells, and the transportation of AsIII takes place via aquaporins32. In the case of S. actus and P. duplex (Group B phytoplankton; Fig. 5), AsV was taken up simultaneously and accumulated inside the cell. The uptake of AsV by microalgae without transformation and their keeping it inside the cell likely occurs due to the detoxification strategies of these freshwater microalgae. The occurrence of this phenomenon in S. actus indicates that this species may be genetically incapable or energetically incompetent to take part in As biotransformation activities33. The subcellular distribution and chemical forms of heavy metals may play important roles in the metal tolerance and detoxification responses in plants34. The ability of A. minutissium and C. aciculare (Group C phytoplankton; Fig. 5) to transform AsV into AsIII or methylated As forms confirm the occurrence of As biotransformation process in these microalgae. In general, bio-reduction occurs in association with the methylation process in response to AsV biotransformation. A study by Murray et al.35 on microalgae, including D. salina and C. vulgaris, provided evidence that this sort of biotransformation of As species could be performed by these microalgae, which was in agreement with the findings for phytoplankton Group C in the present study. Phytoplankton converted AsV to AsIII, and as the incubation period continued the AsIII accumulated in the cells gradually began to undergo the methylation process, mainly to form DMAA, and was then likely excreted into the medium. The quantity of orgAs species present was related to the methylation/demethylation rate and release mechanisms of the microalgae. The presence (or absence) of MMAA in microalgal cell also confirmed the occurrence of the iAs methylation process in them. Therefore, biotransformation diversity among microalgae depends not only on the As speciation in the medium, but also on the As bioaccumulation and biotransformation methods used by each individual phytoplankton species.
The rates of As uptake by the different microalgae used in this study varied, suggesting that the consumption or sequestration of arsenic species depends on the structural and biochemical properties of microalgae, and differs among microalgae belonging to different species or classes. Uptake of As by cells depends on the valence of arsenic, as well as on several biotic and abiotic factors. Relevant biotic factors include the microalgal species and its uptake pathways, mode of detoxification, and whether it has had earlier exposure to As. On the other hand, abiotic factors include the As species, phosphate concentration, pH, and time of exposure. Several studies suggested that living biota (both aquatic and terrestrial) have various methods they can use to detoxify arsenic-like metals and metalloids. These may involve: As elimination from cells36, reduction of AsV to AsIII 37, secretion of polychelatins (e.g., metal-binding proteins)38, and the subsequent methylation of As to form less toxic complexes39. Nevertheless, the detoxification processes carried out by microalgae, due to their impacts on the excretion of AsV, AsIII, or organic As species32, have significant influences on their growth and AsV uptake and elimination.
This study showed that As biotransformation by and toxicity to freshwater phytoplankton was greatly influenced by the chemical species of As present, the type of phytoplankton species considered, and the phosphate concentration in the growth medium. Changes in experiment duration and conditions (such as exposure period and phosphate concentration) altered the behavior and As biotransformation processes carried out by the phytoplankton species. The growth rate of each species varied even when they were treated with the same experimental procedures, suggesting that each species has its own specific As uptake mechanism(s).
A conceptual model of As metabolism in freshwater phytoplankton
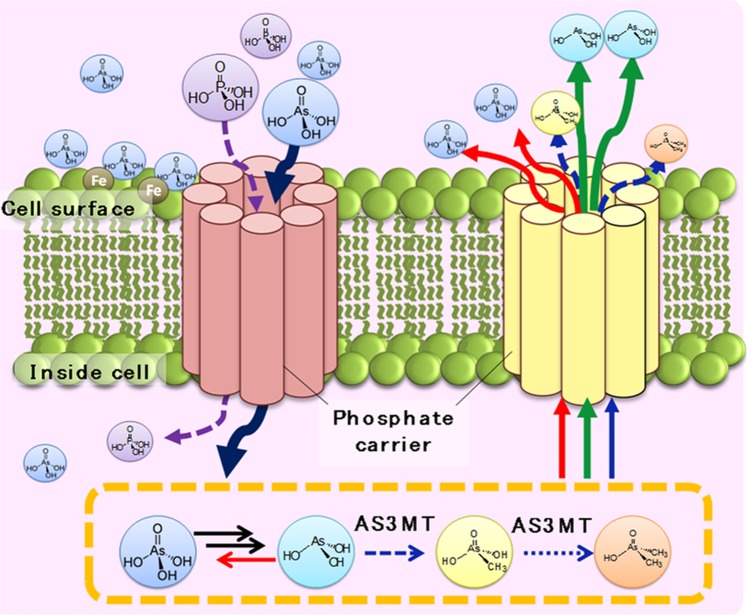
A model of As biotransformation by freshwater phytoplankton based on the experimental results of this study is presented in Fig. 6. In this model, the AsV present in the medium is adsorbed on the cell surface, and is then taken into the cell via the PO43– transport system. This phenomenon happens due to the similar chemical properties of arsenate and phosphate. Arsenate can be actively taken up into the phytoplankton cells by the phosphate transporter pathway40 and competing with phosphate uptake41. This competitive uptake between AsV and phosphate in phytoplankton cells indicates the probable mode of action of the toxicity of As species. The As concentration in the cell proportionally increases with the As substrate concentration in the medium. This suggests that As uptake is influenced by metalloid availability in the environemnt42–44. AsV is reduced to AsIII in the logarithmic growth phase and excreted into the growth medium via active transport. This reduction reaction may be carried out by thiols and/or dithiols, as As is likely to bind with the biochemical components of protein and non-protein thiols39. AsIII is then methylated to form MMAA and DMAA, which then diffuse into the medium, suggesting that the chemical form of As species is changed depending on the As tolerance of the phytoplankton during the transition from the logarithmic growth phase to the stationary phase. Uptake of AsV causes the reduction of phosphate accumulation in the cell, likely due to As phytotoxicity or competitive uptake45. Inside the cell, PO43– groups are replaced by AsV in ATP, which forms an unstable ADP-As complex that interferes with other physiological systems, such as energy flow46.
Figure 6.
Conceptual model of As metabolism in freshwater phytoplankton. Arsenic resistance in this study depended on the kinds of freshwater phytoplankton considered. In the logarithmic growth phase, freshwater phytoplankton took arsenate into their cells and reduced it to arsenite. In the stationary phase, freshwater phytoplankton transformed the chemical forms of the arsenic in the surrounding environment, and then released it from their cells rapidly. The arsenic resistance of freshwater phytoplankton has an interactive relationship with the changing availability of different arsenic species in the hydrosphere.
Uptake of AsIII into the cell occurs via aquaporin nodulin26-like intrinsic proteins (NIPs). Aquaporins are a kind of water channel protein that can transport extracellular water molecules into the cell. Several studies have suggested that AsIII likely interferes with enzymatic reactions47 and the photosynthesis process48. Consequently, pigment, peptide, and lipid profiles can be altered in microalgal cells exposed to inorganic As species49. There are several steps involved in the metabolic transformation of iAs into methylated species, such as mono-, di-, and tri-methylated arsenic compunds50. Among these steps, some are associated with chemical reactions, whereas others are enzymatically catalyzed. It was previously suggested that arsenic methyltransferase (AS3MT) acts as a catalyst that leads to the transformation of iAs into methylated compounds. However, the methylated compounds yielded by this process are actually more reactive and toxic51.
The reduction of AsV to AsIII occurs in the presence of several reductases that use glutaredoxin, glutathione, or thioredoxin as electron donors32,52. The methylation of AsIII was reported to be slower than that of AsV to prevent AsIII from building up inside the cell17. AsIII is excreted less and its concentration gradually declines as a result of ongoing abiotic oxidation. MMAA and DMAA occur inside the cell due to the methylation of AsIII, and their excretion increases their concentrations in the culture medium. The production rates of DMAA and MMAA are comparatively slower than that of AsIII at the logarithmic growth phase, likely due to the lower uptake of AsV at this phase17. The presence of phytochelatins (PCs) in microalgae leads to the binding of As to thio groups, for example glutathione (GSH), and thus PCs play important roles in As complexation and detoxification53. PC synthesis takes place at a rate adequate to bind the As in the cell at lower As concentrations54. The presence of intracellular GSH along with PCs permits the effective accumulation of As inside the cell55. The reduction of AsV to AsIII with GSH as the electron donor in an aquatic medium promotes the formation of arsenotriglutathione complexes. These complexes readily donate AsIII to targets including dithiols56. Hayakawa et al.57 reported that methylation mechanisms could occur via the formation of such complexes. The methyl group of S-adenosylmethionine (SAM) is transferred to As by AS3MT, and then the methylated compound undergoes hydrolysis and is oxidized, and the methylation process then proceeds sequentially. SAM is required for the As methylation reaction, in which it acts as a methyl donor, and if its availability changes this affects the patterns and extent of the As methylation process. The fact that the methylation mechanism of As involves AS3MT indicates that As methylation is an oxidative process. Chemically active oxygen was reported to initiate oxidative stress due to its involvement in the catalytic reaction of methyltransferases58. Free radicals are generated due to the reaction of dimethylarsine with molecular oxygen during the metabolic reduction of DMAAIII. The mechanism of As uptake indicates that the pathway leading from iAs to methylated compounds includes several steps that generate several different intermediates and elements.
Conclusion
This study reported the toxicity, biotransformation, and release of different As species in freshwater phytoplankton, which may help us to better understand the biogeochemistry of As in freshwater systems. Inorganic AsV was taken up by the phytoplankton, biotransformed inside their cells, and then released into the culture medium as AsIII during the exponential growth phase at a high AsV to phosphate ratio. The growth inhibition effects of AsV and AsIII were significantly higher than those of orgAs, DMAAV, and AB on the tested freshwater phytoplankton. The six freshwater phytoplankton strains examined could be categorized based on their As biotransformation patterns as follows: (i) those efficient in the biotransformation of AsIII into AsV; (ii) those not efficient in As biotransformation, which rather maintained AsV inside their cells; and (iii) those efficient in the biotransformation of iAs into methylAs species (methylation) and complex orgAs species. These results reflect the differential bioaccumulation and biotransformation of As species by these phytoplankton inside their cells and their excretion of these As species into the environment. Further work is needed to assess whether the toxicity of As species differs for other aquatic microorganisms such as zooplankton, to better understand the influence of As on freshwater ecosystem dynamics.
Materials and Methods
Reagents
Commercially available products were used as reagents without further purification in this study. Special-grade NaOH (NakaraiTesque, Kyoto, Japan) and HCl (Kanto Chemical, Japan) were used to adjust the pH of reagents and media. Special-grade disodium hydrogen formate heptahydrate (AsV) and arsenic trioxide (AsIII) (Wako, Osaka, Japan), sodium cacodylate (DMAAV) (NacaraiTesque, Kyoto, Japan), and arsenobetaine (AB) (Tri Chemical, Yamanashi, Japan) were used for the modification of culture media, and 4-(2-hydroxyethyl)-1-piperazinyl ethanesulfonic acid (HEPES; NacalaiTesque, Kyoto, Japan) was used as a buffer reagent in culture media. KH2PO4 was purchased from Wako (Osaka, Japan) and ethylenediamine-N,N,N′,N′-tetraacetic acid (EDTA; >98.0%) was obtained from Dojindo, Japan. These reagents were diluted to the desired concentration in ultrapure water. Water was purified using ultrapure water production equipment (arium pro UV, Sartorius StedimBiotech GmbH) with a resistivity of 18.2 MΩ.
Phytoplankton
Six strains of clonal axenic freshwater phytoplankton, namely Achnanthidium minutissimum, Botryococcus braunii, Scenedesmus actus, Staurastrum paradoxum, Pediastrum duplex, and Closterium aciculare, were used in the present study. The strains of A. minutissimum, B. braunii, S. actus, S. paradoxum, and P. duplex used were obtained from the National Institute for Environmental Studies, Japan. The tested strain of C. aciculare (isolated from Lake Biwa) was provided by Dr. Kanako Naito of Prefectural University of Hiroshima, Japan. The bioconcentration factors of the phytoplankton obtained under the same experimental conditions used in this study are provided in Table 1.
Table 1.
Bioconcentration factor (BCFa) of freshwater phytoplankton used in this study.
| Arsenic treatments (nmol L−1) | Culture period (days) | Chlorophyceae | Charophyceae | Bacilariophyceae | |||
|---|---|---|---|---|---|---|---|
| Botryococcus braunii | Scenedesmus actus | Pediastrum duplex | Closterium aciculare | Staurastrum paradoxum | Achnanthidium minutissimum | ||
| Bioconcentration factors | |||||||
| 20 | 7 | 86.0 | 13.8 | 14.5 | 9.9 | 84.6 | 4.4 |
| 20 | 21 | 28.3 | 6.9 | 12.0 | 9.8 | 37.0 | 3.8 |
| 1000 | 7 | 12.8 | 1.5 | 1.0 | 0.3 | 8.9 | 0.3 |
| 1000 | 21 | 0.8 | 0.1 | 0.3 | 0.1 | 3.6 | 0.1 |
a, where, CB is the intracellular arsenic concentration per 1.0 g of cell dry weight and CW is the arsenic concentration in culture media.
A. minutissimum is distinguished from other monoraphid diatoms by its small size, linear-lanceolate shape, and radiate striae. Cells are solitary or form very short chains, and are often attached to the substrate by a stalk. This species’ distribution is biased towards alkaline waters, but it also appears in acidic waters59. It is widely adaptable to organic pollution, and dominates in rivers polluted by heavy metals59.
B. braunii is a pyramid-shaped planktonic green microalga that belongs to the family Botryococcaceae. This microalga inhabits freshwater, with cells with a diameter of 10–20 µm that form aggregated colonies. This species is able to produce hydrocarbons (particularly triterpenes), which comprise around 30–40% of its dry weight60. This organism synthesizes oil in its cells that is secreted extracellularly. The oil produced by B. braunii is expected to someday be used as an alternative fuel to gasoline.
S. actus is a green microalga belonging to the family Scenedesmaceae that has lanceolate cells. It always forms colonies, with colonies of 4 (or 2, 8, or 16) cells often connected in a line. It is widely distributed in freshwater environments, such as paddy fields, ponds, and swamps, as well as in soil. Its cells adhere to one another via the cell wall, and their positions do not change. In addition, the whole body may be wrapped in agar with extracellular polysaccharide secretions. The cells constituting the colonies have no flagella and are not motile.
S. paradoxum is a green microalga in the family Desmidiaceae with solitary floating cells. There is a constriction at the center of the cell, which divides each cell into two half-cells (desmids). The shape of the cell as seen from above (top-view) is a regular polygon. Four long arms extend from the central point. It is extremely common in various freshwater areas, such as lakes, ponds, paddy fields, rivers, and so on.
P. duplex is a species of freshwater green microalgae in the family Hydrodictyaceae. It forms colonies with specific numbers of cells (8 to 32 cells). The cell bodies are polygonal, granulated, and have horn-like projections. One cell has two protrusions, and there is a wide gap between the cells. The colonies are as large as single-celled algae, with a diameter that reaches tens to hundreds of microns, and the colonies have limited motility. This microalga is widely distributed in freshwater environments, such as paddy fields, ponds, and swamps. Most of these microalgae are free-floating, but there are also benthic forms.
C. aciculare is a crescent-shaped, unicellular, freshwater microalga. It can be found in almost all freshwater environments, from still-water ponds to running waters. There are 2 (rarely 4) chloroplasts in each cell, and they are divided in the center.
Pre-culture and maintenance
CSi medium (Table 2) was used for the maintenance of the six studied freshwater phytoplankton strains. For the growth experiment, the phosphate concentrations of the CSi medium were adjusted to 1.0 μmol L−1 or 50 μmol L−1, and then media were modified by supplementation with arsenic solutions. The culture medium and apparatus (tips, bottles, vessels, and micropipettes) were sterilized separately at 121 °C for 30 min using an autoclave (MLS 3780, Sanyo Electric, Japan). They were then placed in a clean bench (NK Clean Bench, VSF-1300A, Nippon Medical Equipment Co., Japan) under UV irradiation for 20 min. Before using the phytoplankton in the trials, the cultures were maintained in identical media for 1–2 weeks in polycarbonate bottles (Nalge, USA) until they reached at least the exponential growth phase in a temperature- and light-controlled incubator (Koitotron3HN-35MLA, Koito Industries, Ltd. Japan). Experimental cultures were grown at 25 °C under a 12:12 h light/dark photoperiod, at a light intensity of 50 μE m−2 s−1 provided by cool white fluorescent lights. The axenic nature of the phytoplankton cultures was verified by performing the 4′,6-diamidino-2-phenylindole (DAPI) test and by the examination of cells under an epifluorescence microscope61.
Table 2.
Chemical composition of CSi culture medium for freshwater phytoplankton used in this study.
| Nutrients/Chemicals | Concentrations |
|---|---|
| Ca(NO3)2·4H2O | 635 mmol L−1 |
| KNO3 | 989 mmol L−1 |
| KH2PO4 | 213 mmol L−1 |
| MgSO4·7H2O | 162 mmol L−1 |
| MnCl2·4H2O | 0.55 mmol L−1 |
| ZnSO4·7H2O | 0.23 mmol L−1 |
| CoCl2·6H2O | 0.05 mmol L−1 |
| Na2MoO4·2H2O | 2.73 mmol L−1 |
| FeCl3 | 1.31 mmol L−1 |
| Na2EDTA·2H2O | 8.06 mmol L−1 |
| Na2SiO3·9H2O | 10 mg mL−1 |
| Vitamin | 1.0 × 10−6 mg mL−1 |
| 4-(2-hydroxyethyl)-1-piperazine ethane sulphonate | 50 mg mL−1 |
Growth experiments with various As concentrations
Phytoplankton cells acclimated to culture media at the exponential growth phase were incubated in 60-mL capacity polycarbonate vessels containing 60 mL of sterilized CSi culture medium (Table 2). The culture medium was then modified by changing the concentration of phosphate (added as KH2PO4) to encourage the uptake of arsenic species. Two different arsenic concentration treatments, high- arsenic ([As]0 = 1.0 μmol L−1) and low-arsenic ([As]0 = 20 nmol L−1), were used in the experiment, in which As was provided in the form of NaH2AsO4. Initially, the cell density in the culture medium was less than 4.6 × 103 cells mL−1. After incubating the phytoplankton for three days, four species of arsenic (AsV, AsIII, DMAAV, and AB) were added to the culture medium. The cultures were grown for 30 days. Phytoplankton growth was measured spectrophotometrically using a UV-VIS (ultraviolet-visible) spectrophotometer at 540 nm. The cell number was estimated with an established cell density-to-absorbance ratio. The number of cells was counted directly under a microscope. The growth rate (%) was defined by the following equation:
where ODSample is the optical density of phytoplankton at 540 nm in arsenic-containing media and ODControl is the optical density of phytoplankton in arsenic-free media after seven days of cultivation.
Sample processing
On a pre-specified day, samples were collected and filtered through 0.45-μm membrane filters under low vacuum pressure (<30 mm Hg). The filtrate sample was then stored in a cool and dark place after adding 0.50 mL of 1.0 mol L−1 HCl to them. After that, 20 mL of 3.0 mol L−1 HCl was added to the sample, and the mixture was heated on a hot plate at 100 °C for 3 h to extract the arsenic both inside and outside of the freshwater phytoplankton cells. Thereafter, the mixture volume was adjusted to 40 mL with ultrapure water, and it was also adjusted to the same concentration as that of 1.3 mol L−1 HCl (pH = 0).
Analysis of arsenic speciation in microalgal samples
A hydride generation technique was used for the determination of arsenic species in culture media according to Hasegawa et al.62. The technique was a combination of a flame atomic absorption spectrophotometer (AAS, 170-50 A, Hitachi, Japan) and hydride generation device followed by cold trapping8,63. Concentrations of iAs (AsV + AsIII), MMAA, and DMAA were analyzed by adding 5.0 mL of 0.20 mol L−1 EDTA·4Na and 5.0 mol L−1 HCl to 40 mL of the sample solution3. For AsIII, 5.0 mL of 0.20 mol L−1 EDTA·4Na and 0.5 mol L−1 KH2PO4 was added to 40 mL of the sample solution. The arsenic species detected were recorded in the form of a chromatogram using a data processing device (Chromato-PRO, Runtime Instruments, Tokyo, Japan), and their concentrations were determined based on the heights of the peaks obtained. A representative chromatogram of the occurrence and separation of peaks for each of the arsenic species is given in Fig. 7. The limit of detections (LODs) for the iAs (AsV + AsIII), MMAA, and DMAA were 0.3, 0.8 and 0.7 nmol L−1, respectively. The precisions as relative standard deviation (RSD, n = 5) for 20 nmol L−1 of iAs (AsV + AsIII), MMAA, and DMAA were 2.2, 1.2 and 1.4%, respectively. The accuracy of the analysis was checked using the certified standard reference material 1573a (tomato leaf from NIST, USA)64. The recovery of As concentration was 95.0% of the certified value.
Figure 7.
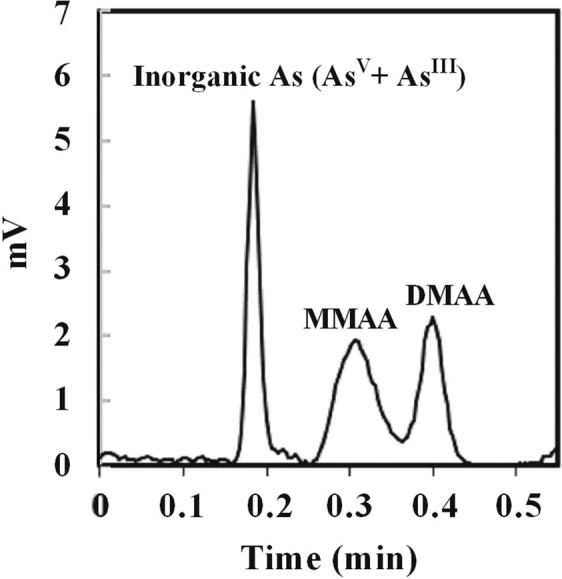
Chromatogram showing occurrence and separation of peaks representing each arsenic species for 20 nmol L−1 concentration sample.
Determination of the total arsenic concentrations in cell samples
The total concentrations of arsenic in the freshwater microalgae were determined by inductively coupled plasma mass spectrometry (ICP-MS, SPQ 9000, SEIKO, Japan). Filter paper containing microalgal cells was digested by a microwave digestion system (Multiwave 3000, Anton Paar) using the Sea 1-h method. After that, digested liquors were co-washed with 15 mL of ultrapure water and transferred to heat-resistant DigiTUBEs (DigiPREP Jr, SCP SCIENCE). Tubes were then heated on hot plates at 100 °C for 6–7 h. After evaporation, 2 mL of deionized water was added to the samples, and they were then subjected to ICP-MS for the quantification of the total arsenic content in their cells. The operational conditions of the ICP-MS were the following: a high-frequency output of 1.2 kW; plasma gas flow rate of 16 L min−1; auxiliary gas flow rate of 1.0 L min−1; nebulizer gas flow rate of 1.0 L min−1; and sample replacement time of 10 s.
The mean values in different treatments were compared using Duncan’s multiple range test using the statistical program SPSS 22.0 for Windows (SPSS Inc., USA).
Acknowledgements
The research was partially supported by Grants-in-Aid for Scientific Research (15H05118 and 18H03399) from the Japan Society for the Promotion of Science. The authors are thankful to English-speaking editors at Editage (www.editage.jp) for language editing of the manuscript.
Author Contributions
H.H. conceived the study; H.H. and M.A.R. designed the experiment and interpreted the results; E.I., Y.O., A.S.M. and T.M. conducted the experiment; R.I.P. performed data analyses and prepared the figures; and H.H., M.A.R. and R.I.P. wrote the manuscript; all authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hiroshi Hasegawa, Email: hhiroshi@se.kanazawa-u.ac.jp.
Rimana Islam Papry, Email: papry015@gmail.com.
M. Azizur Rahman, Email: rahmanmazizur@gmail.com.
References
- 1.Rahman MA, Hasegawa H, Lim RP. Bioaccumulation, biotransformation and trophic transfer of arsenic in the aquatic food chain. Environ. Res. 2012;116:118–135. doi: 10.1016/j.envres.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Rahman MA, Hassler C. Is arsenic biotransformation a detoxification mechanism for microorganisms? Aquat. Toxicol. 2014;146:212–219. doi: 10.1016/j.aquatox.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Hasegawa H, et al. Seasonal changes of arsenic speciation in lake waters in relation to eutrophication. Sci. Total Environ. 2010;408:1684–1690. doi: 10.1016/j.scitotenv.2009.11.062. [DOI] [PubMed] [Google Scholar]
- 4.Mamun M. Abdullah Al, Omori Yoshiki, Papry Rimana Islam, Kosugi Chika, Miki Osamu, Rahman Ismail M. M., Mashio Asami S., Maki Teruya, Hasegawa Hiroshi. Bioaccumulation and biotransformation of arsenic by the brown macroalga Sargassum patens C. Agardh in seawater: effects of phosphate and iron ions. Journal of Applied Phycology. 2019;31(4):2669–2685. doi: 10.1007/s10811-018-1721-x. [DOI] [Google Scholar]
- 5.Price A, et al. Distribution of arsenic species in an open seagrass ecosystem: Relationship to trophic groups, habitats and feeding zones. Environ. Chem. 2012;9:77–88. doi: 10.1071/EN11105. [DOI] [Google Scholar]
- 6.Rahman MA, Hasegawa H. Arsenic in freshwater systems: Influence of eutrophication on occurrence, distribution, speciation, and bioaccumulation. Appl. Geochem. 2012;27:304–314. doi: 10.1016/j.apgeochem.2011.09.020. [DOI] [Google Scholar]
- 7.Navratilova J, Raber G, Fisher SJ, Francesconi KA. Arsenic cycling in marine systems: degradation of arsenosugars to arsenate in decomposing algae, and preliminary evidence for the formation of recalcitrant arsenic. Environ. Chem. 2011;8:44–51. doi: 10.1071/EN10107. [DOI] [Google Scholar]
- 8.Papry RI, et al. Arsenic biotransformation potential of six marine diatom species: effect of temperature and salinity. Sci. Rep. 2019;9:10226. doi: 10.1038/s41598-019-46551-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye J, Rensing C, Rosen BP, Zhu YG. Arsenic biomethylation by photosynthetic organisms. Trends Plant Sci. 2012;17:155–162. doi: 10.1016/j.tplants.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oremland RS, Stolz JF. The ecology of arsenic. Science (New York, N.Y.) 2003;300:939–944. doi: 10.1126/science.1081903. [DOI] [PubMed] [Google Scholar]
- 11.Levy JL, et al. Toxicity, biotransformation, and mode of action of arsenic in two freshwater microalgae (Chlorella sp. and Monoraphidium arcuatum) Environ. Toxicol. Chem. 2005;24:2630–2639. doi: 10.1897/04-580r.1. [DOI] [PubMed] [Google Scholar]
- 12.Maeda S, Arima H, Ohki A, Naka K. The association mode of arsenic accumulated in the freshwater alga Chlorella vulgaris. Appl. Organomet. Chem. 1992;6:393–397. doi: 10.1002/aoc.590060414. [DOI] [Google Scholar]
- 13.Mateos LM, Ordóñez E, Letek M, Gil JA. Corynebacterium glutamicum as a model bacterium for the bioremediation of arsenic. Int. Microbiol. 2010;9:207–215. [PubMed] [Google Scholar]
- 14.Hasegawa H, et al. Biosynthesis and release of methylarsenic compounds during the growth of freshwater algae. Chemosphere. 2001;43:265–272. doi: 10.1016/S0045-6535(00)00137-5. [DOI] [PubMed] [Google Scholar]
- 15.Suhendrayatna AO, Maeda S. Biotransformation of arsenite in freshwater food chain models. Appl. Organomet. Chem. 2001;15:277–284. doi: 10.1002/aoc.139. [DOI] [Google Scholar]
- 16.Yin XX, et al. Biotransformation and volatilization of arsenic by three photosynthetic cyanobacteria. Plant Physiol. 2011;156:1631–1638. doi: 10.1104/pp.111.178947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hellweger FL, Farley KJ, Lall U, Di Toro DM. Greedy algae reduce arsenate. Limnol. Oceanolgr. 2003;48:2275–2288. doi: 10.4319/lo.2003.48.6.2275. [DOI] [Google Scholar]
- 18.Hughes MF. Arsenic toxicity and potential mechanisms of action. Toxicol. Lett. 2002;133:1–16. doi: 10.1016/S0378-4274(02)00084-X. [DOI] [PubMed] [Google Scholar]
- 19.Caumette G, Koch I, Estrada E, Reimer KJ. Arsenic speciation in plankton organisms from contaminated lakes: Transformations at the base of the freshwater food chain. Environ. Sci. Technol. 2011;45:9917–9923. doi: 10.1021/es2025092. [DOI] [PubMed] [Google Scholar]
- 20.Hirata S, Toshimitsu H. Determination of arsenic species and arsenosugars in marine samples by HPLC–ICP–MS. Anal. Bioanal. Chem. 2005;383:454–460. doi: 10.1007/s00216-005-3413-z. [DOI] [PubMed] [Google Scholar]
- 21.Llorente-Mirandes T, Ruiz-Chancho MJ, Barbero M, Rubio R, López-Sánchez JF. Measurement of arsenic compounds in littoral zone algae from the Western Mediterranean Sea: Occurrence of arsenobetaine. Chemosphere. 2010;81:867–875. doi: 10.1016/j.chemosphere.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Miyashita S, Fujiwara S, Tsuzuki M, Kaise T. Rapid biotransformation of arsenate into oxo-arsenosugars by a freshwater unicellular green alga, Chlamydomonas reinhardtii. Biosci. Biotech. Bioch. 2011;75:522–530. doi: 10.1271/bbb.100751. [DOI] [PubMed] [Google Scholar]
- 23.Šlejkovec Z, Kápolna E, Ipolyi I, van Elteren JT. Arsenosugars and other arsenic compounds in littoral zone algae from the Adriatic Sea. Chemosphere. 2006;63:1098–1105. doi: 10.1016/j.chemosphere.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Hasegawa H, et al. Effect of eutrophication on the distribution of arsenic species in eutrophic and mesotrophic lakes. Sci. Total Environ. 2009;407:1418–1425. doi: 10.1016/j.scitotenv.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 25.Sohrin Y, Matsui M, Kawashima M, Hojo M, Hasegawa H. Arsenic biogeochemistry affected by eutrophication in Lake Biwa, Japan. Environ. Sci. Technol. 1997;31:2712–2720. doi: 10.1021/es960846w. [DOI] [Google Scholar]
- 26.Rahman MA, et al. Toxicity of arsenic species to three freshwater organisms and biotransformation of inorganic arsenic by freshwater phytoplankton (Chlorella sp. CE-35) Ecotoxicol. Environ. Saf. 2014;106:126–135. doi: 10.1016/j.ecoenv.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Wang N-X, et al. Toxicity and bioaccumulation kinetics of arsenate in two freshwater green algae under different phosphate regimes. Water Res. 2013;47:2497–2506. doi: 10.1016/j.watres.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 28.Sharma VK, Sohn M. Aquatic arsenic: Toxicity, speciation, transformations, and remediation. Environ. Int. 2009;35:743–759. doi: 10.1016/j.envint.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Ohki A, Maeda S. Biotransformation of arsenite in freshwater food‐chain models. Appl. Organomet. Chem. 2001;15:277–284. doi: 10.1002/aoc.139. [DOI] [Google Scholar]
- 30.Baker J, Wallschläger D. The role of phosphorus in the metabolism of arsenate by a freshwater green alga, Chlorella vulgaris. J. Environ. Sci. 2016;49:169–178. doi: 10.1016/j.jes.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Akter, K. F., Owens, G., Davey, D. E. & Naidu, R. Arsenic speciation and toxicity in biological systems. Rev. Environ. Contam. Toxicol., 97–149 (2005). [DOI] [PubMed]
- 32.Zhao FJ, Ma JF, Meharg AA, McGrath SP. Arsenic uptake and metabolism in plants. New Phytol. 2009;181:777–794. doi: 10.1111/j.1469-8137.2008.02716.x. [DOI] [PubMed] [Google Scholar]
- 33.Rose M, et al. Arsenic in seaweed—Forms, concentration and dietary exposure. Food Chem. Toxicol. 2007;45:1263–1267. doi: 10.1016/j.fct.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Fu X, et al. Subcellular distribution and chemical forms of cadmium in Phytolacca americana L. J. Hazard. Mater. 2011;186:103–107. doi: 10.1016/j.jhazmat.2010.10.122. [DOI] [PubMed] [Google Scholar]
- 35.Murray LA, Raab A, Marr IL, Feldmann J. Biotransformation of arsenate to arsenosugars by Chlorella vulgaris. Appl. Organomet. Chem. 2003;17:669–674. doi: 10.1002/aoc.498. [DOI] [Google Scholar]
- 36.Meharg A, Macnair M. Suppression of the high affinity phosphate uptake system: a mechanism of arsenate tolerance in Holcus lanatus L. J. Exp. Bot. 1992;43:519–524. doi: 10.1093/jxb/43.4.519. [DOI] [Google Scholar]
- 37.Rosen BP. Families of arsenic transporters. Trends Microl. 1999;7:207–212. doi: 10.1016/S0966-842X(99)01494-8. [DOI] [PubMed] [Google Scholar]
- 38.De Vos CR, Vonk MJ, Vooijs R, Schat H. Glutathione depletion due to copper-induced phytochelatin synthesis causes oxidative stress in Silene cucubalus. Plant Physiol. 1992;98:853–858. doi: 10.1104/pp.98.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cullen WR, Li H, Pergantis SA, Eigendorf GK, Harrison LG. The methylation of arsenate by a marine alga Polyphysa peniculus in the presence of L-methionine-methyl-d3. Chemosphere. 1994;28:1009–1019. doi: 10.1016/0045-6535(94)90017-5. [DOI] [Google Scholar]
- 40.Reed ST, Ayala-Silva T, Dunn CB, Gordon GG. Effects of arsenic on nutrient accumulation and distribution in selected ornamental plants. Agric. Sci. 2015;6:1513. [Google Scholar]
- 41.Meharg AA, Hartley‐Whitaker J. Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species. New Phytol. 2002;154:29–43. doi: 10.1046/j.1469-8137.2002.00363.x. [DOI] [Google Scholar]
- 42.Smith SE, Christophersen HM, Pope S, Smith FA. Arsenic uptake and toxicity in plants: integrating mycorrhizal influences. Plant Soil. 2010;327:1–21. doi: 10.1007/s11104-009-0089-8. [DOI] [Google Scholar]
- 43.Maeda S, Kusadome K, Arima H, Ohki A, Naka K. Biomethylation of arsenic and its excretion by the alga Chlorella vulgaris. Appl. Organomet. Chem. 1992;6:407–413. doi: 10.1002/aoc.590060416. [DOI] [Google Scholar]
- 44.Budd K, Craig SR. Resistance to arsenate toxicity in the blue-green alga Synechococcus leopoliensis. Can. J. Bot. 1981;59:1518–1521. doi: 10.1139/b81-207. [DOI] [Google Scholar]
- 45.Wang J, et al. Mechanisms of arsenic hyperaccumulation in Pteris vittata. uptake kinetics, interactions with phosphate, and arsenic speciation. Plant Physiol. 2002;130:1552–1561. doi: 10.1104/pp.008185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ullrich-Eberius, C., Sanz, A. & Novacky, A. Evaluation of arsenate-and vanadate-associated changes of electrical membrane potential and phosphate transport in Lemna gibba G1. J. Exp. Bot., 119–128 (1989).
- 47.Planas D, Healey FP. Effects of arsenate on growth and phosphorus metabolism of phytoplankton. J. Phycol. 1978;14:337–341. doi: 10.1111/j.1529-8817.1978.tb00309.x. [DOI] [Google Scholar]
- 48.Blanck H, Wängberg S-Å. Induced community tolerance in marine periphyton established under arsenate stress. Can. J. Fish. Aquat. Sci. 1988;45:1816–1819. doi: 10.1139/f88-213. [DOI] [Google Scholar]
- 49.Miazek K, Iwanek W, Remacle C, Richel A, Goffin D. Effect of metals, metalloids and metallic nanoparticles on microalgae growth and industrial product biosynthesis: a review. Int. J. Mol. Sci. 2015;16:23929–23969. doi: 10.3390/ijms161023929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas DJ, et al. Arsenic (+3 oxidation state) methyltransferase and the methylation of arsenicals in the invertebrate chordate Ciona intestinalis. Toxicol. Sci. 2009;113:70–76. doi: 10.1093/toxsci/kfp250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas DJ, Styblo M, Lin S. The cellular metabolism and systemic toxicity of arsenic. Toxicol. Appl. Pharnacol. 2001;176:127–144. doi: 10.1006/taap.2001.9258. [DOI] [PubMed] [Google Scholar]
- 52.Yin X, Wang L, Duan G, Sun G. Characterization of arsenate transformation and identification of arsenate reductase in a green alga Chlamydomonas reinhardtii. J. Environ. Sci. 2011;23:1186–1193. doi: 10.1016/S1001-0742(10)60492-5. [DOI] [PubMed] [Google Scholar]
- 53.Pawlik-Skowrońska B, Pirszel J, Kalinowska R, Skowroński T. Arsenic availability, toxicity and direct role of GSH and phytochelatins in As detoxification in the green alga Stichococcus bacillaris. Aquat. Toxicol. 2004;70:201–212. doi: 10.1016/j.aquatox.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Morelli E, Mascherpa MC, Scarano G. Biosynthesis of phytochelatins and arsenic accumulation in the marine microalga Phaeodactylum tricornutum in response to arsenate exposure. Biometals. 2005;18:587–593. doi: 10.1007/s10534-005-2998-1. [DOI] [PubMed] [Google Scholar]
- 55.Yamaoka Y, Takimura O, Fuse H, Murakami K. Effect of glutathione on arsenic accumulation by Dunaliella salina. Appl. Organomet. Chem. 1999;13:89–94. doi: 10.1002/(SICI)1099-0739(199902)13:2<89::AID-AOC803>3.0.CO;2-L. [DOI] [Google Scholar]
- 56.Delnomdedieu M, Basti MM, Otvos JD, Thomas DJ. Transfer of arsenite from glutathione to dithiols: a model of interaction. Chem. Res. Toxicol. 1993;6:598–602. doi: 10.1021/tx00035a002. [DOI] [PubMed] [Google Scholar]
- 57.Hayakawa T, Kobayashi Y, Cui X, Hirano S. A new metabolic pathway of arsenite: arsenic–glutathione complexes are substrates for human arsenic methyltransferase Cyt19. Arch. Toxicol. 2005;79:183–191. doi: 10.1007/s00204-004-0620-x. [DOI] [PubMed] [Google Scholar]
- 58.Hu Y, Jin X, Snow ET. Effect of arsenic on transcription factor AP-1 and NF-κB DNA binding activity and related gene expression. Toxicol. Lett. 2002;133:33–45. doi: 10.1016/S0378-4274(02)00083-8. [DOI] [PubMed] [Google Scholar]
- 59.Watanabe K, et al. Symbiotic association in Chlorella culture. FEMS Microbiol. Ecol. 2005;51:187–196. doi: 10.1016/j.femsec.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 60.Metzger P, Largeau C. Botryococcus braunii: a rich source for hydrocarbons and related ether lipids. Appl. Microbiol. Biotechnol. 2005;66:486–496. doi: 10.1007/s00253-004-1779-z. [DOI] [PubMed] [Google Scholar]
- 61.Bohlool, B. B. & Schmidt, E. L. In Advances in Microbial Ecology (ed M. Alexander) 203–241 (Springer, 1980).
- 62.Hasegawa H, Sohrin Y, Matsui M, Hojo M, Kawashima M. Speciation of arsenic in natural waters by solvent extraction and hydride generation atomic absorption spectrometry. Anal. Chem. 1994;66:3247–3252. doi: 10.1021/ac00091a039. [DOI] [Google Scholar]
- 63.Mamun MAA, et al. Comparative biotransformation and detoxification potential of arsenic by three macroalgae species in seawater: Evidence from laboratory culture studies. Chemosphere. 2019;228:117–127. doi: 10.1016/j.chemosphere.2019.04.056. [DOI] [PubMed] [Google Scholar]
- 64.Rahman MA, Hasegawa H, Kadohashi K, Maki T, Ueda K. Hydroxyiminodisuccinic acid (HIDS): A novel biodegradable chelating ligand for the increase of iron bioavailability and arsenic phytoextraction. Chemosphere. 2009;77:207–213. doi: 10.1016/j.chemosphere.2009.07.032. [DOI] [PubMed] [Google Scholar]