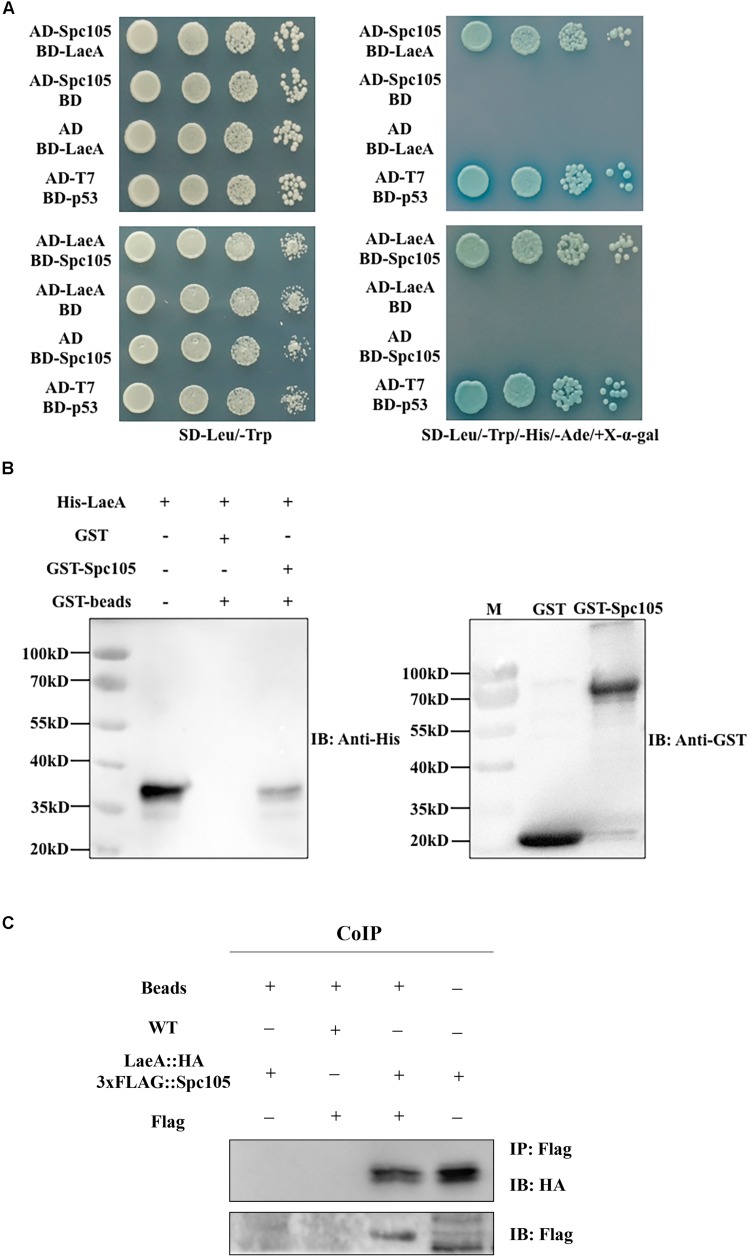
FIGURE 9.
Spc105 interacts with LaeA in the Y2H and GST pull-down assays. (A) Y2H assays to determine protein–protein interactions. Yeast cells were grown in liquid selective medium overnight and diluted serially. Four microliters of serially diluted yeast cells were spotted on selective synthetic dropout media SD/-Leu/-Trp/-His/-Ade/ + X-α-gal and incubated at 30°C for 3–5 days. The SD/-Leu/-Trp plate is non-selective and served as the loading control. (B) GST pull-down assay of the interaction between Spc105 and LaeA in vitro. Recombinant GST and GST-Spc105 were incubated with recombinant His6-LaeA and subsequently purified by glutathione magnetic beads. Note that we tried to but failed to express the full length of recombinant GST-Spc105, and here is the truncated version (800 aa-1541 aa) of Spc105. Immunoblot analysis was performed to detect the presence of His6-LaeA using an anti-His-tag antibody. (C) Co-IP of LaeA:HA and 3xFLAG:Spc105. Affinity purification assays from Flag-tagged Spc 105 strain in the background of HA-tagged LaeA were performed with Flag-Trap magnetic beads. The coimmunoprecipitated proteins were analyzed by the anti-HA antibody.