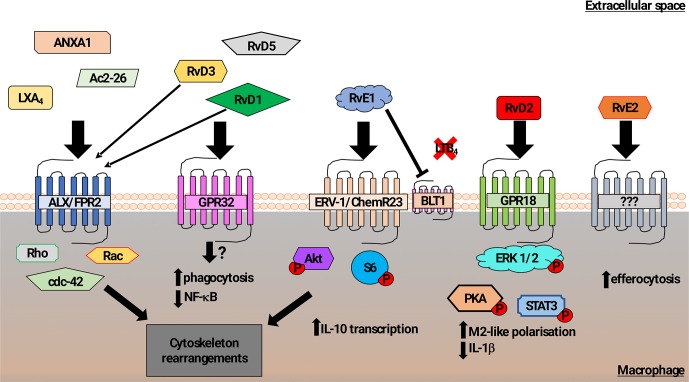
Figure 1.
Schematic representation of mechanisms promoting macrophage phagocytosis of AC by lipid mediators. In addition to a role in recognition of N-formylated peptides that are generated during bacterial and mitochondrial protein synthesis, the formyl peptide receptor 2 (ALX/FPR2) also binds to Lipoxin A4, Annexin A1, and Ac2-26 (N-terminal part of Annexin A1) to increase macrophage phagocytosis of AC (Godson et al., 2000; Maderna et al., 2002; Scannell et al., 2007; Maderna et al., 2010). Signaling following ALX/FPR2 binding by these ligands was shown to induce rearrangements in the actin cytoskeleton in a Rho, Rac, and cdc42-dependent manner. GPR32 is thought to be the main receptor for the resolvin D family members 1, 3, and 5 (RvD1,3,5) that acts to promote phagocytosis of AC and reduce NF-κB activity. RvD1 and RvD3 were also found to bind ALX/FPR2 with high affinity and induce macrophage phagocytosis (Arita et al., 2007; Krishnamoorthy et al., 2010, Krishnamoorthy et al., 2012; Dalli et al., 2013b). The resolving E family member 1 (RvE1) increased macrophage phagocytosis of AC via binding to ERV-1/ChemR23. Phosphorylation of Akt and S6 proteins induced cytoskeletal rearrangements as well as promotion of transcription of the anti-inflammatory cytokine IL-10 (Laguna-Fernandez et al., 2018). RvE1 can also competitively bind to the leukotriene B4 receptor BLT4, acting to reduce pro-inflammatory signaling (Arita et al., 2007; Ohira et al., 2010; Laguna-Fernandez et al., 2018). Resolvin D2 (RvD2) mediated activation of GPR18 induced an M2-like macrophage phenotype exhibiting increased phagocytosis via a mechanism involving phosphorylation of the ERK, PKA, and STAT3 (Fredman and Serhan, 2011; Dalli et al., 2013b). Although resolving E2 (RvE2) was also reported to induce macrophage phagocytosis, the pathway that controls this effect has not been clearly identified (Oh et al., 2012).