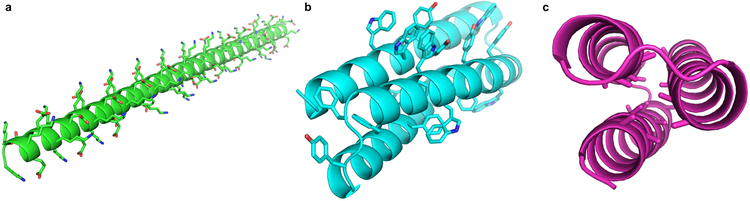
Extended Data Figure 1. Initial top-ranking Foldit player designs.
When challenged to design a protein with only the talaris2013 score function (and no additional rules), Foldit players discovered low-energy models that are unlikely to fold as designed. a, An extended α-helix, composed entirely of lysine and glutamate, has very favorable energies for hydrogen-bonding, electrostatic, and backbone torsions, but is unlikely to fold cooperatively into a single stable structure. This type of design is discouraged with the “Core Exists” rule. b, Due to their greater surface area, large aromatic sidechains can make more interactions than smaller aliphatic sidechains, even when under-packed or solvent-exposed. This type of design is discouraged with the “Residue Interaction Energy” rule. c, A design with an alanine- and glycine-saturated core can make favorable van der Waals interactions between closely packed backbone atoms; however, the burial of these small sidechains is associated with a weaker hydrophobic effect, and the lack of interdigitation allows exchange between multiple conformations with similar core packing energies (i.e. “molten globule” behavior). These designs are discouraged with the “Secondary Structure Design” rule.