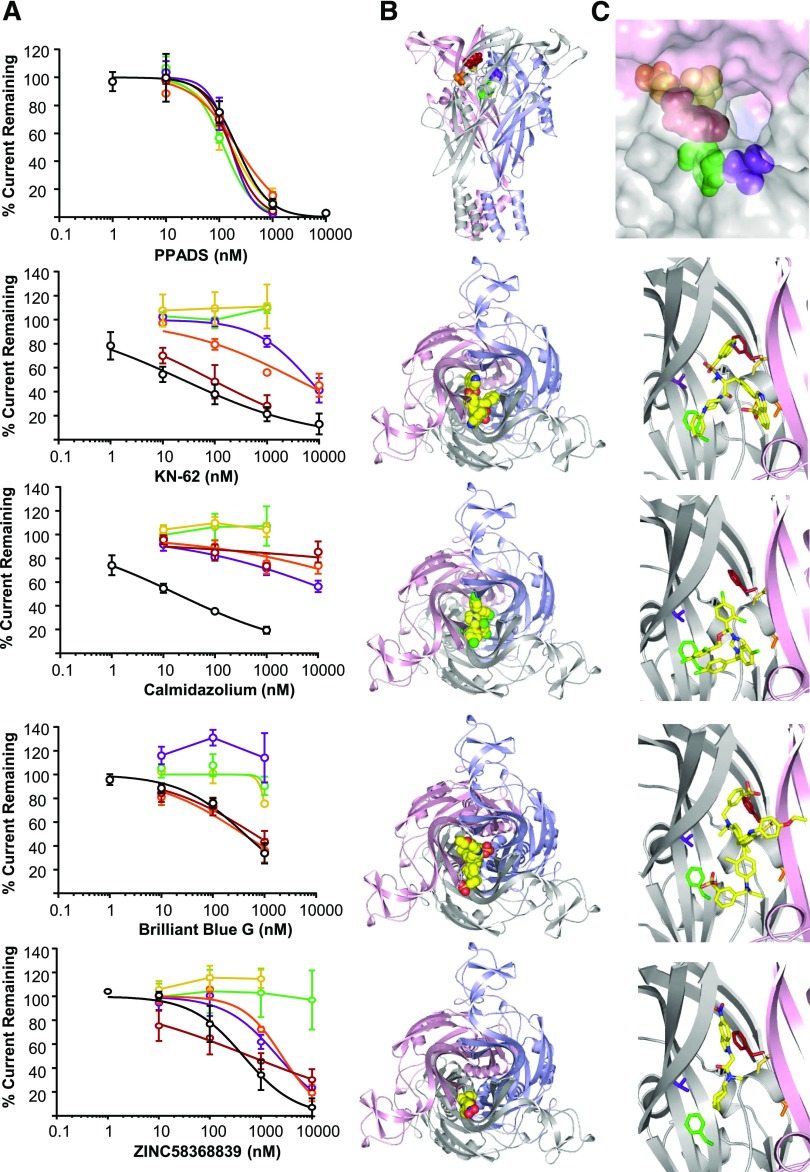
Fig. 5.
Use of intersubunit allosteric pocket “signature mutants” to investigate the site of action for brilliant blue G, KN-62, calmidazolium, ZINC58368839, and PPADS. (A) Concentration-dependent inhibition by P2X7 antagonists of response to an EC90 concentration of ATP for P2X7-2Nβ (black), F88A (firebrick), T90V (orange), D92A (yellow), F103A (green), and V312A (purple). (B) Top panel: P2X7R overview with “signature mutants” residues shown as spheres; colors as in (A). Other panels: View from the top of the extracellular domains along the central axis perpendicular to the membrane. The P2X7R model is shown as cartoon with the three subunits highlighted in light blue, light pink, and gray with the docked pose of the antagonist shown as spheres. (C) Top panel: Surface representation of entrance to allosteric pocket. Other panels: Zoom into the proposed binding site, one subunit [light blue in (B)] is omitted for clarity. Residues F88, T90, D92, F103, and V312 and the respective antagonist are shown as sticks [colors as in (A) and (B)].