Figure 3.
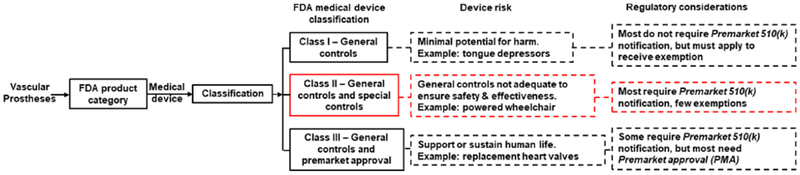
FDA framework for the regulation of medical devices highlighting the regulatory pathway for vascular prostheses currently on the market. Specifically, current vascular prostheses are categorized as Class II medical devices with “special controls”and should align with the nonbinding recommendations provided by the FDA in the “Guidance Document for Vascular Prostheses 510(k) Submissions - Guidance for Industry and FDA staff’ [116, 157]
