Abstract
Chikungunya virus (CHIKV) is an arbovirus transmitted by Aedes mosquitos in tropical and subtropical regions across the world. After decades of sporadic outbreaks, it re-emerged in Africa, Asia, India Ocean and America suddenly, causing major regional epidemics recently and becoming a notable global health problem. Infection by CHIKV results in a spectrum of clinical diseases including an acute self-limiting febrile illness in most individuals, a chronic phase of recurrent join pain in a proportion of patients, and long-term arthralgia for months to years for the unfortunate few. No specific anti-viral drugs or licensed vaccines for CHIKV are available so far. A better understanding of virus-host interactions is essential for the development of therapeutics and vaccines. To this end, we reviewed the existing knowledge on CHIKV’s epidemiology, clinical presentation, molecular virology, diagnostic approaches, host immune response, vaccine development, and available animal models. Such a comprehensive overview, we believe, will shed lights on the promises and challenges in CHIKV vaccine development.
Keywords: chikungunya virus (CHIKV), epidemiology, molecular virology, host immune response, vaccine development
Acknowledgments
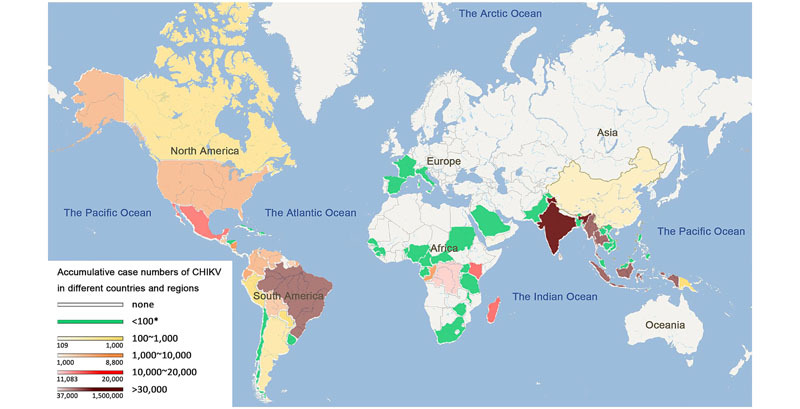
We thank Dr. Chao Zhang (Zerun Biotech Co., Ltd., China) for his help in editing the global heat map of accumulative CHIKV cases. This work is supported in part by the National Key Program Project Grant from MOST #2016YFC1201000.
Footnotes
This article is published with open access at Springerlink.com
These authors contributed equally to this work.
References
- Aggarwal M, Sharma R, Kumar P, Parida M, Tomar S. Kinetic characterization of trans-proteolytic activity of Chikungunya virus capsid protease and development of a FRET-based HTS assay. Sci Rep. 2015;5:14753. doi: 10.1038/srep14753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard E, Solignat M, Gay B, Chazal N, Higgs S, Devaux C, Briant L. Endocytosis of chikungunya virus into mammalian cells: role of clathrin and early endosomal compartments. PLoS One. 2010;5:e11479. doi: 10.1371/journal.pone.0011479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgherini G, Poubeau P, Jossaume A, Gouix A, Cotte L, Michault A, Arvin-Berod C, Paganin F. Persistent arthralgia associated with chikungunya virus: a study of 88 adult patients on reunion island. Clin Infect Dis. 2008;47:469–475. doi: 10.1086/590003. [DOI] [PubMed] [Google Scholar]
- Brandler S, Ruffie C, Combredet C, Brault JB, Najburg V, Prevost MC, Habel A, Tauber E, Despres P, Tangy F. A recombinant measles vaccine expressing chikungunya virus-like particles is strongly immunogenic and protects mice from lethal challenge with chikungunya virus. Vaccine. 2013;31:3718–3725. doi: 10.1016/j.vaccine.2013.05.086. [DOI] [PubMed] [Google Scholar]
- Brighton SW, Simson IW. A destructive arthropathy following Chikungunya virus arthritis-a possible association. Clin Rheumatol. 1984;3:253–258. doi: 10.1007/BF02030766. [DOI] [PubMed] [Google Scholar]
- Brito CA, Sohsten AK, Leitao CC, Brito RC, Valadares LD, Fonte CA, Mesquita ZB, Cunha RV, Luz K, Leao HM, Brito CM, Frutuoso LC. Pharmacologic management of pain in patients with Chikungunya: a guideline. Rev Soc Bras Med Trop. 2016;49:668–679. doi: 10.1590/0037-8682-0279-2016. [DOI] [PubMed] [Google Scholar]
- Broeckel R, Haese N, Messaoudi I, Streblow DN. Nonhuman Primate Models of Chikungunya Virus Infection and Disease (CHIKV NHP Model) Pathogens. 2015;4:662–681. doi: 10.3390/pathogens4030662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Centers for Disease Controlprevention CDC. Chikungunya Virus. 2016. pp. 1571–1572. [Google Scholar]
- Cao-Lormeau VM, Musso D. 2014. Emerging arboviruses in the Pacific. Lancet, 384 [DOI] [PubMed]
- Cassadou S, Boucau S, Petit-Sinturel M, Huc P, Leparc-Goffart I, Ledrans M. Emergence of chikungunya fever on the French side of Saint Martin island, October to December 2013. Euro Surveill. 2014;19:20752. doi: 10.2807/1560-7917.es2014.19.13.20752. [DOI] [PubMed] [Google Scholar]
- Cecilia D, Kakade M, Alagarasu K, Patil J, Salunke A, Parashar D, Shah PS. Development of a multiplex real-time RTPCR assay for simultaneous detection of dengue and chikungunya viruses. Arch Virol. 2015;160:323–327. doi: 10.1007/s00705-014-2217-x. [DOI] [PubMed] [Google Scholar]
- Chang LJ, Dowd KA, Mendoza FH, Saunders JG, Sitar S, Plummer SH, Yamshchikov G, Sarwar UN, Hu Z, Enama ME, Bailer RT, Koup RA, Schwartz RM, Akahata W, Nabel GJ, Mascola JR, Pierson TC, Graham BS, Ledgerwood JE. Safety and tolerability of chikungunya virus-like particle vaccine in healthy adults: a phase 1 dose-escalation trial. The Lancet. 2014;384:2046–2052. doi: 10.1016/S0140-6736(14)61185-5. [DOI] [PubMed] [Google Scholar]
- Charrel R d, Lamballerie X, Raoult D. Chikungunya outbreaks- the globalization of vectorborne diseases. N Engl J Med. 2007;356:769–771. doi: 10.1056/NEJMp078013. [DOI] [PubMed] [Google Scholar]
- Couderc T, Chretien F, Schilte C, Disson O, Brigitte M, Guivel-Benhassine F, Touret Y, Barau G, Cayet N, Schuffenecker I, Despres P, Arenzana-Seisdedos F, Michault A, Albert ML, Lecuit M. A mouse model for Chikungunya: young age and inefficient type-I interferon signaling are risk factors for severe disease. PLoS Pathog. 2008;4:e29. doi: 10.1371/journal.ppat.0040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Zure AD, Berkowitz NM, Graham BS, Ledgerwood JE. Whole-Inactivated and Virus-Like Particle Vaccine Strategies for Chikungunya Virus. J Infect Dis. 2016;214:S497–S499. doi: 10.1093/infdis/jiw352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diallo D, Sall AA, Buenemann M, Chen R, Faye O, Diagne CT, Faye O, Ba Y, Dia I, Watts D, Weaver SC, Hanley KA, Diallo M. Landscape ecology of sylvatic chikungunya virus and mosquito vectors in southeastern Senegal. PLoS Negl Trop Dis. 2012;6:e1649. doi: 10.1371/journal.pntd.0001649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman R, Tacket CO, Wasserman SS, Bodison SA, Perry JG, Mangiafico JA. Phase II safety and immunogenicity study of live chikungunya virus vaccine TSI-GSD-218. Am J Trop Med Hyg. 2000;62:681–685. doi: 10.4269/ajtmh.2000.62.681. [DOI] [PubMed] [Google Scholar]
- Edwards CJ, Welch SR, Chamberlain J, Hewson R, Tolley H, Cane PA, Lloyd G. Molecular diagnosis and analysis of Chikungunya virus. J Clin Virol. 2007;39:271–275. doi: 10.1016/j.jcv.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Erasmus JH, Needham J, Raychaudhuri S, Diamond MS, Beasley DW, Morkowski S, Salje H F, Salas I, Kim DY, Frolov I, Nasar F, Weaver SC. Utilization of an Eilat Virus- Based Chimera for Serological Detection of Chikungunya Infection. PLoS Negl Trop Dis. 2015;9:e0004119. doi: 10.1371/journal.pntd.0004119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erasmus JH, Rossi SL, Weaver SC. Development of Vaccines for Chikungunya Fever. J Infect Dis. 2016;214:S488–S496. doi: 10.1093/infdis/jiw271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsell K, Suomalainen M, Garoff H. Structure-function relation of the NH2-terminal domain of the Semliki Forest virus capsid protein. J Virol. 1995;69:1556–1563. doi: 10.1128/jvi.69.3.1556-1563.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourie ED, Morrison JG. Rheumatoid arthritic syndrome after chikungunya fever. S Afr Med J. 1979;56:130–132. [PubMed] [Google Scholar]
- Gardner J, Anraku I, Le TT, Larcher T, Major L, Roques P, Schroder WA, Higgs S, Suhrbier A. Chikungunya virus arthritis in adult wild-type mice. J Virol. 2010;84:8021–8032. doi: 10.1128/JVI.02603-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons DL, Vaney MC, Roussel A, Vigouroux A, Reilly B, Lepault J, Kielian M, Rey FIA. Conformational change and protein-protein interactions of the fusion protein of Semliki Forest virus. Nature. 2004;427:320–325. doi: 10.1038/nature02239. [DOI] [PubMed] [Google Scholar]
- Goo L, Dowd KA, Lin TY, Mascola JR, Graham BS, Ledgerwood JE, Pierson TC. A Virus-Like Particle Vaccine Elicits Broad Neutralizing Antibody Responses in Humans to All Chikungunya Virus Genotypes. J Infect Dis. 2016;214:1487–1491. doi: 10.1093/infdis/jiw431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorchakov R, Wang E, Leal G, Forrester NL, Plante K, Rossi SL, Partidos CD, Adams AP, Seymour RL, Weger J, Borland EM, Sherman MB, Powers AM, Osorio JE, Weaver SC. Attenuation of Chikungunya virus vaccine strain 181/clone 25 is determined by two amino acid substitutions in the E2 envelope glycoprotein. J Virol. 2012;86:6084–6096. doi: 10.1128/JVI.06449-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goupil BA, McNulty MA, Martin MJ, McCracken MK, Christofferson RC, Mores CN. Novel Lesions of Bones and Joints Associated with Chikungunya Virus Infection in Two Mouse Models of Disease: New Insights into Disease Pathogenesis. PLoS One. 2016;11:e0155243. doi: 10.1371/journal.pone.0155243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandadam M, Caro V, Plumet S, Thiberge JM, Souares Y, Failloux AB, Tolou HJ, Budelot M, Cosserat D, Leparc-Goffart I, Despres P. Chikungunya virus, southeastern France. Emerg Infect Dis. 2011;17:910–913. doi: 10.3201/eid1705.101873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz NG. Critical review of the vector status of Aedes albopictus. Med Vet Entomol. 2004;18:215–227. doi: 10.1111/j.0269-283X.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- Guo R, Peng Z, Song T, He J, Zhong H, Li L, Liang W. Current infection status and epidemic risk analysis of Dengue fever and Chikungunya in Guangdong province, from 1990 to 2012. Chin J Epidemiol. 2014;35:167–169. [PubMed] [Google Scholar]
- Guzman MG, Harris E. Dengue. Lancet. 2015;385:453–465. doi: 10.1016/S0140-6736(14)60572-9. [DOI] [PubMed] [Google Scholar]
- Hallengard D, Kakoulidou M, Lulla A, Kummerer BM, Johansson DX, Mutso M, Lulla V, Fazakerley JK, Roques P, Le Grand R, Merits A, Liljestrom P. Novel attenuated Chikungunya vaccine candidates elicit protective immunity in C57BL/6 mice. J Virol. 2014;88:2858–2866. doi: 10.1128/JVI.03453-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawman DW, Fox JM, Ashbrook AW, May NA, Schroeder KM, Torres RM, Crowe JE, Jr, Dermody TS, Diamond MS, Morrison TE. Pathogenic Chikungunya Virus Evades B Cell Responses to Establish Persistence. Cell Rep. 2016;16:1326–1338. doi: 10.1016/j.celrep.2016.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawman DW, Stoermer KA, Montgomery SA, Pal P, Oko L, Diamond MS, Morrison TE. Chronic joint disease caused by persistent Chikungunya virus infection is controlled by the adaptive immune response. J Virol. 2013;87:13878–13888. doi: 10.1128/JVI.02666-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Her Z, Malleret B, Chan M, Ong EK, Wong SC, Kwek DJ, Tolou H, Lin RT, Tambyah PA, Renia L, Ng LF. Active infection of human blood monocytes by Chikungunya virus triggers an innate immune response. J Immunol. 2010;184:5903–5913. doi: 10.4049/jimmunol.0904181. [DOI] [PubMed] [Google Scholar]
- Hoornweg TE, van Duijl-Richter MK, Ayala Nunez NV, Albulescu IC, van Hemert MJ, Smit JM. Dynamics of Chikungunya Virus Cell Entry Unraveled by Single-Virus Tracking in Living Cells. J Virol. 2016;90:4745–4756. doi: 10.1128/JVI.03184-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huits R, Okabayashi T, Cnops L, Barbe B, Van Den Berg R, Bartholomeeusen K, Arien KK, Jacobs J, Bottieau E, Nakayama EE, Shioda T, Van Esbroeck M. Clin Microbiol Infect. 2017. Diagnostic accuracy of a rapid E1-antigen test for chikungunya virus infection in a reference setting. [DOI] [PubMed] [Google Scholar]
- Hussain KM, Lee RC, Ng MM, Chu JJ. Establishment of a Novel Primary Human Skeletal Myoblast Cellular Model for Chikungunya Virus Infection and Pathogenesis. Sci Rep. 2016;6:21406. doi: 10.1038/srep21406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BW, Goodman CH, Holloway K d, Salazar PM, Valadere AM, Drebot MA. Evaluation of Commercially Available Chikungunya Virus Immunoglobulin M Detection Assays. Am J Trop Med Hyg. 2016;95:182–192. doi: 10.4269/ajtmh.16-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BW, Russell BJ, Goodman CH. Laboratory Diagnosis of Chikungunya Virus Infections and Commercial Sources for Diagnostic Assays. J Infect Dis. 2016;214:S471–S474. doi: 10.1093/infdis/jiw274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonathan J, Lindsey E, Jun P. Sci Transl Med. 2017. Therapy with CTLA4-Ig and an antiviral monoclonal antibody controls chikungunya virus arthritis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josseran L, Paquet C, Zehgnoun A, Caillere N, Le Tertre A, Solet JL, Ledrans M. Chikungunya disease outbreak, Reunion Island. Emerg Infect Dis. 2006;12:1994–1995. doi: 10.3201/eid1212.060710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam YW, Simarmata D, Chow A, Her Z, Teng TS, Ong EK, Renia L, Leo YS, Ng LF. Early appearance of neutralizing immunoglobulin G3 antibodies is associated with chikungunya virus clearance and long-term clinical protection. J Infect Dis. 2012;205:1147–1154. doi: 10.1093/infdis/jis033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap RS, Morey SH, Ramteke SS, Chandak NH, Parida M, Deshpande PS, Purohit HJ, Taori GM, Daginawala HF. Diagnosis of Chikungunya fever in an Indian population by an indirect enzyme-linked immunosorbent assay protocol based on an antigen detection assay: a prospective cohort study. Clin Vaccine Immunol. 2010;17:291–297. doi: 10.1128/CVI.00326-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AH, Morita K, Parquet MD, Mdel C, Hasebe F, Mathenge EG, Igarashi A. J GenVirol. 2002. Complete nucleotide sequence of chikungunya virus and evidence for an internal polyadenylation site; pp. 3075–3084. [DOI] [PubMed] [Google Scholar]
- Khan M, Dhanwani R, Kumar JS, Rao PV, Parida M. Comparative evaluation of the diagnostic potential of recombinant envelope proteins and native cell culture purified viral antigens of Chikungunya virus. J Med Virol. 2014;86:1169–1175. doi: 10.1002/jmv.23752. [DOI] [PubMed] [Google Scholar]
- Khan M, Dhanwani R, Rao PV, Parida M. Subunit vaccine formulations based on recombinant envelope proteins of Chikungunya virus elicit balanced Th1/Th2 response and virusneutralizing antibodies in mice. Virus Res. 2012;167:236–246. doi: 10.1016/j.virusres.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Klimstra WB, Nangle EM, Smith MS, Yurochko AD, Ryman KD. DC-SIGN and L-SIGN Can Act as Attachment Receptors for Alphaviruses and Distinguish between Mosquito Cell- and Mammalian Cell-Derived Viruses. Journal of Virology. 2003;77:12022–12032. doi: 10.1128/JVI.77.22.12022-12032.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labadie K, Larcher T, Joubert C, Mannioui A, Delache B, Brochard P, Guigand L, Dubreil L, Lebon P, Verrier B, de Lamballerie X, Suhrbier A, Cherel Y, Le Grand R, Roques P. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J Clin Invest. 2010;120:894–906. doi: 10.1172/JCI40104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WW, Teo TH, Her Z, Lum FM, Kam YW, Haase D, Renia L, Rotzschke O, Ng LF. J Virol. 2015. Expanding regulatory T cells alleviates chikungunya virus-induced pathology in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum FM, Teo TH, Lee WW, Kam YW, Renia L, Ng LF. An essential role of antibodies in the control of Chikungunya virus infection. J Immunol. 2013;190:6295–6302. doi: 10.4049/jimmunol.1300304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti-Carvajal A, Ramon-Pardo P, Javelle E, Simon F, Aldighieri S, Horvath H, Rodriguez-Abreu J, Reveiz L. Interventions for treating patients with chikungunya virus infection-related rheumatic and musculoskeletal disorders: A systematic review. PLoS One. 2017;12:e0179028. doi: 10.1371/journal.pone.0179028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi I, Vomaske J, Totonchy T, Kreklywich CN, Haberthur K, Springgay L, Brien JD, Diamond MS, Defilippis VR, Streblow DN. Chikungunya virus infection results in higher and persistent viral replication in aged rhesus macaques due to defects in anti-viral immunity. PLoS Negl Trop Dis. 2013;7:e2343. doi: 10.1371/journal.pntd.0002343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz SW, Martina BE, van den Doel P, Geertsema C, Osterhaus AD, Vlak JM, Pijlman GP. Chikungunya virus-like particles are more immunogenic in a lethal AG129 mouse model compared to glycoprotein E1 or E2 subunits. Vaccine. 2013;31:6092–6096. doi: 10.1016/j.vaccine.2013.09.045. [DOI] [PubMed] [Google Scholar]
- Miner JJ, Diamond MS. Zika Virus Pathogenesis and Tissue Tropism. Cell Host Microbe. 2017;21:134–142. doi: 10.1016/j.chom.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabayashi T, Sasaki T, Masrinoul P, Chantawat N, Yoksan S, Nitatpattana N, Chusri S M, Vargas RE, Grandadam M, Brey PT, Soegijanto S, Mulyantno KC, Churrotin S, Kotaki T, Faye O, Faye O, Sow A, Sall AA, Puiprom O, Chaichana P, Kurosu T, Kato S, Kosaka M, Ramasoota P, Ikuta K. Detection of chikungunya virus antigen by a novel rapid immunochromatographic test. J Clin Microbiol. 2015;53:382–388. doi: 10.1128/JCM.02033-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan American Health Organization PAHO. Geographic Spread of Chikungunya in the Americas 2013–2017. 2017. [Google Scholar]
- Panning M, Grywna K, van Esbroeck M, Emmerich P, Drosten C. Chikungunya fever in travelers returning to Europe from the Indian Ocean region, 2006. Emerg Infect Dis. 2008;14:416–422. doi: 10.3201/eid1403.070906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parola P, de Lamballerie X, Jourdan J, Rovery C, Vaillant V, Minodier P, Brouqui P, Flahault A, Raoult D, Charrel RN. Novel chikungunya virus variant in travelers returning from Indian Ocean islands. Emerg Infect Dis. 2006;12:1493–1499. doi: 10.3201/eid1210.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince HE, Seaton BL, Matud JL, Batterman HJ. Chikungunya virus RNA and antibody testing at a National Reference Laboratory since the emergence of Chikungunya virus in the Americas. Clin Vaccine Immunol. 2015;22:291–297. doi: 10.1128/CVI.00720-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran V, Kaur P, Kanagasabai K, Vadivoo S, Murhekar MV. Persistent arthralgia among Chikungunya patients and associated risk factors in Chennai, South India. J Postgrad Med. 2014;60:3–6. doi: 10.4103/0022-3859.128795. [DOI] [PubMed] [Google Scholar]
- Ramsauer K, Schwameis M, Firbas C, Müllner M, Putnak RJ, Thomas SJ, Desprès P, Tauber E, Jilma B, Tangy F. Immunogenicity, safety, and tolerability of a recombinant measlesvirus- based chikungunya vaccine: a randomised, double-blind, placebo-controlled, active-comparator, first-in-man trial. The Lancet Infectious Diseases. 2015;15:519–527. doi: 10.1016/S1473-3099(15)70043-5. [DOI] [PubMed] [Google Scholar]
- Reddy V, Ravi V, Desai A, Parida M, Powers AM, Johnson BW. Utility of IgM ELISA, TaqMan real-time PCR, reverse transcription PCR, and RT-LAMP assay for the diagnosis of Chikungunya fever. J Med Virol. 2012;84:1771–1778. doi: 10.1002/jmv.23406. [DOI] [PubMed] [Google Scholar]
- Renault P, Josseran L, Pierre V. Chikungunya-related fatality rates, Mauritius, India, and Reunion Island. Emerg Infect Dis. 2008;14:1327. doi: 10.3201/eid1408.080201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli AC, Panning M, Cordioli P, Fortuna C, Boros S, Magurano F, Silvi G, Angelini P, Dottori M, Ciufolini MG, Majori GC, Cassone A g Cs. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370:1840–1846. doi: 10.1016/S0140-6736(07)61779-6. [DOI] [PubMed] [Google Scholar]
- Robinson MC. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952-53. I. Clinical features. Trans R Soc Trop Med Hyg. 1955;49:28–32. doi: 10.1016/0035-9203(55)90080-8. [DOI] [PubMed] [Google Scholar]
- Roy CJ, Adams AP, Wang E, Plante K, Gorchakov R, Seymour RL, Vinet-Oliphant H, Weaver SC. Chikungunya vaccine candidate is highly attenuated and protects nonhuman primates against telemetrically monitored disease following a single dose. J Infect Dis. 2014;209:1891–1899. doi: 10.1093/infdis/jiu014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd PA, Wilson J, Gardner J, Larcher T, Babarit C, Le TT, Anraku I, Kumagai Y, Loo YM, Gale M Jr, Akira S, Khromykh AA, Suhrbier A. Interferon response factors 3 and 7 protect against Chikungunya virus hemorrhagic fever and shock. J Virol. 2012;86:9888–9898. doi: 10.1128/JVI.00956-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilte C, Staikowsky F, Couderc T, Madec Y, Carpentier F, Kassab S, Albert ML, Lecuit M, Michault A. Chikungunya virus-associated long-term arthralgia: a 36-month prospective longitudinal study. PLoS Negl Trop Dis. 2013;7:e2137. doi: 10.1371/journal.pntd.0002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwameis M, Buchtele N, Wadowski PP, Schoergenhofer C, Jilma B. Chikungunya vaccines in development. Hum Vaccin Immunother. 2016;12:716–731. doi: 10.1080/21645515.2015.1101197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz O, Albert ML. Biology and pathogenesis of chikungunya virus. Nat Rev Microbiol. 2010;8:491–500. doi: 10.1038/nrmicro2368. [DOI] [PubMed] [Google Scholar]
- Shukla J, Khan M, Tiwari M, Sannarangaiah S, Sharma S, Rao PV, Parida M. Development and evaluation of antigen capture ELISA for early clinical diagnosis of chikungunya. Diagn Microbiol Infect Dis. 2009;65:142–149. doi: 10.1016/j.diagmicrobio.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Silva LA, Dermody TS. Chikungunya virus: epidemiology, replication, disease mechanisms, and prospective intervention strategies. J Clin Invest. 2017;127:737–749. doi: 10.1172/JCI84417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon F, Javelle E, Cabie A, Bouquillard E, Troisgros O, Gentile G, Leparc-Goffart I, Hoen B, Gandjbakhch F, Rene-Corail P, Franco JM, Caumes E, Combe B, Poiraudeau S, Gane-Troplent F, Djossou F, Schaerverbeke T, Criquet-Hayot A, Carrere P, Malvy D, Gaillard P, Wendling D, Societe de pathologie infectieuse de langue f. French guidelines for the management of chikungunya (acute and persistent presentations). November 2014. Med Mal Infect. 2015;45:243–263. doi: 10.1016/j.medmal.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Simon F, Javelle E, Oliver M, Leparc-Goffart I, Marimoutou C. Chikungunya virus infection. Curr Infect Dis Rep. 2011;13:218–228. doi: 10.1007/s11908-011-0180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh I, Helenius A. Role of ribosomes in Semliki Forest virus nucleocapsid uncoating. J Virol. 1992;66:7049–7058. doi: 10.1128/jvi.66.12.7049-7058.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TJ, Cheng RH, Olson NH, Peterson P, Chase E, Kuhn RJ, Baker TS. Immunology. 1995. Putative receptor binding sites on alphaviruses as visualized by cryoelectron microscopy; pp. 10648–10652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder AJ, Mukhopadhyay S. The alphavirus E3 glycoprotein functions in a clade-specific manner. J Virol. 2012;86:13609–13620. doi: 10.1128/JVI.01805-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JE, Kulcsar KA, Schultz KL, Riley CP, Neary JT, Marr S, Jose J, Griffin DE, Kuhn RJ. Functional characterization of the alphavirus TF protein. J Virol. 2013;87:8511–8523. doi: 10.1128/JVI.00449-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solignat M, Gay B, Higgs S, Briant L, Devaux C. Replication cycle of chikungunya: a re-emerging arbovirus. Virology. 2009;393:183–197. doi: 10.1016/j.virol.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourisseau M, Schilte C, Casartelli N, Trouillet C, Guivel-Benhassine F, Rudnicka D, Sol-Foulon N, et al. Characterization of Reemerging Chikungunya Virus. PLoS Pathog. 2007;3:e89. doi: 10.1371/journal.ppat.0030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples JE, Breiman RF, Powers AM. Chikungunya fever: an epidemiological review of a re-emerging infectious disease. Clin Infect Dis. 2009;49:942–948. doi: 10.1086/605496. [DOI] [PubMed] [Google Scholar]
- Strauss JH, Strauss EG. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhrbier A, Jaffar-Bandjee MC, Gasque P. Arthritogenic alphaviruses-an overview. Nat Rev Rheumatol. 2012;8:420–429. doi: 10.1038/nrrheum.2012.64. [DOI] [PubMed] [Google Scholar]
- Teo TH, Lum FM, Claser C, Lulla V, Lulla A, Merits A, Renia L, Ng LF. A pathogenic role for CD4+ T cells during Chikungunya virus infection in mice. J Immunol. 2013;190:259–269. doi: 10.4049/jimmunol.1202177. [DOI] [PubMed] [Google Scholar]
- Townson H, Nathan MB. Resurgence of chikungunya. Trans R Soc Trop Med Hyg. 2008;102:308–309. doi: 10.1016/j.trstmh.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Tretyakova I, Hearn J, Wang E, Weaver S, Pushko P. DNA vaccine initiates replication of live attenuated chikungunya virus in vitro and elicits protective immune response in mice. J Infect Dis. 2014;209:1882–1890. doi: 10.1093/infdis/jiu114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villamil-Gomez WE, Gonzalez-Camargo O, Rodriguez-Ayubi J, Zapata-Serpa D, Rodriguez-Morales AJ. Dengue, chikungunya and Zika co-infection in a patient from Colombia. J Infect Public Health. 2016;9:684–686. doi: 10.1016/j.jiph.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Volk SM, Chen R, Tsetsarkin KA, Adams AP, Garcia TI, Sall AA, Nasar F, Schuh AJ, Holmes EC, Higgs S, Maharaj PD, Brault AC, Weaver SC. Genome-scale phylogenetic analyses of chikungunya virus reveal independent emergences of recent epidemics and various evolutionary rates. J Virol. 2010;84:6497–6504. doi: 10.1128/JVI.01603-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JE, Vaney MC, Duquerroy S, Vonrhein C, Girard-Blanc C, Crublet E, Thompson A, Bricogne G, Rey FA. Glycoprotein organization of Chikungunya virus particles revealed by Xray crystallography. Nature. 2010;468:709–712. doi: 10.1038/nature09555. [DOI] [PubMed] [Google Scholar]
- World Health Organization WHO. Chikungunya. 2017. [Google Scholar]
- World Health Organization WHO. Outbreak news. Chikungunya and dengue, south-west Indian Ocean. Wkly Epidemiol Rec. 2006;81:106–108. [PubMed] [Google Scholar]
- Wahid B, Ali A, Rafique S, Idrees M. Global expansion of chikungunya virus: mapping the 64-year history. Int J Infect Dis. 2017;58:69–76. doi: 10.1016/j.ijid.2017.03.006. [DOI] [PubMed] [Google Scholar]
- Wauquier N, Becquart P, Nkoghe D, Padilla C, Ndjoyi-Mbiguino A, Leroy EM. The acute phase of Chikungunya virus infection in humans is associated with strong innate immunity and T CD8 cell activation. J Infect Dis. 2011;204:115–123. doi: 10.1093/infdis/jiq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C, Berberich E, von Rhein C, Henss L, Hildt E, Schnierle BS. Identification of Functional Determinants in the Chikungunya Virus E2 Protein. PLoS Negl Trop Dis. 2017;11:e0005318. doi: 10.1371/journal.pntd.0005318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weger-Lucarelli J, Aliota MT, Wlodarchak N, Kamlangdee A, Swanson R, Osorio JE. Dissecting the Role of E2 Protein Domains in Alphavirus Pathogenicity. J Virol. 2015;90:2418–2433. doi: 10.1128/JVI.02792-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B, Nitschko H, Ghattas I, Wright R, Schlesinger S. Evidence for specificity in the encapsidation of Sindbis virus RNAs. J Virol. 1989;63:5310–5318. doi: 10.1128/jvi.63.12.5310-5318.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintachai P, Wikan N, Kuadkitkan A, Jaimipuk T, Ubol S, Pulmanausahakul R, Auewarakul P, Kasinrerk W, Weng WY, Panyasrivanit M, Paemanee A, Kittisenachai S, Roytrakul S, Smith DR. Identification of prohibitin as a Chikungunya virus receptor protein. J Med Virol. 2012;84:1757–1770. doi: 10.1002/jmv.23403. [DOI] [PubMed] [Google Scholar]
- Zeller H V, Bortel W, Sudre B. Chikungunya: Its History in Africa and Asia and Its Spread to New Regions in 2013-2014. J Infect Dis. 2016;214:S436–S440. doi: 10.1093/infdis/jiw391. [DOI] [PubMed] [Google Scholar]


