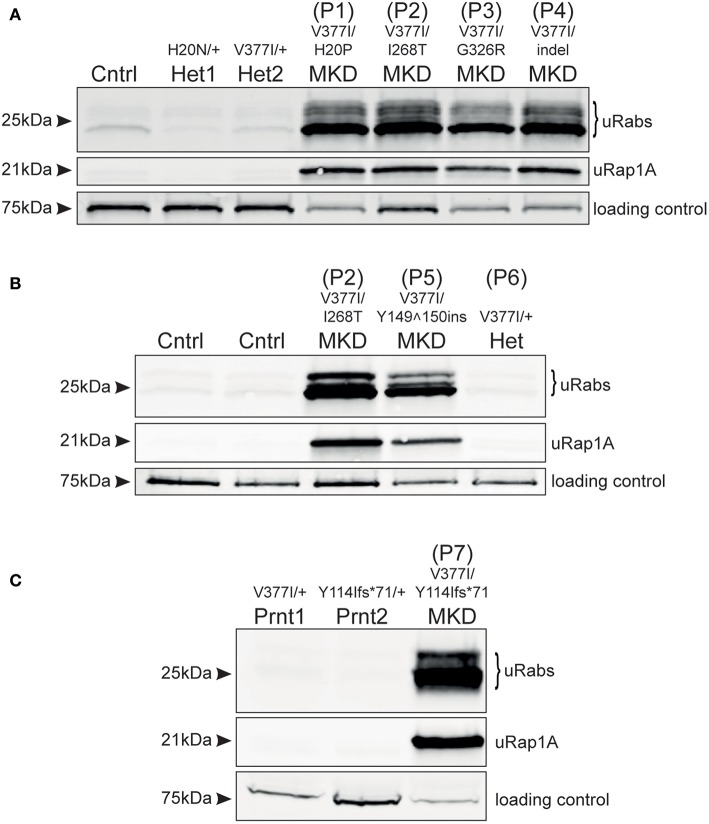
Figure 1.
Defective protein prenylation in PBMCs is detected consistently in patients with MKD. (A) PBMCs from four adult, compound heterozygous MKD patients (P1–P4) have clear accumulation of unprenylated Rab GTPases (uRabs) and unprenylated Rap1A (uRap1) compared to a healthy control (Cntrl) and 2 heterozygous individuals (Het1,2). (B) PBMCs from a young adult patient with MKD (P5) show accumulation of unprenylated Rab and Rap1A GTPases compared to 2 healthy controls (Cntrl) and a heterozygous (Het) individual (P6) with undiagnosed inflammatory disease. Patient P2 is also shown alongside for comparison. (C) A child (P7) with MKD shows a severe defective in Rab and Rap1A prenylation in PBMCs, compared to both unaffected, heterozygous parents (Prnt1,2). Seventy-five kilodalton endogenous biotinylated protein was used as a loading control in all of the blots.