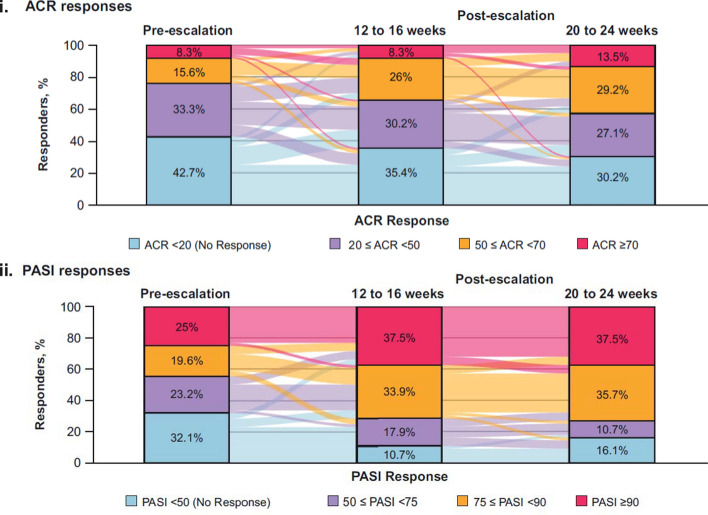
Fig. 3.
i ACR and ii PASI responses before and after dose escalation. Pre-escalation is defined as the last assessment done on or before the patient was administered the 300 mg dose; patients with both pre- and all post-dose escalation assessment data available are included in the analysis; non-responders are considered in the dose escalation subset; PASI responses are reported only in patients with at least 3% body surface area affected with psoriasis at baseline. Colors flowing in the background indicate the proportion of patients changing the response over time. M is the number of patients evaluated (M = 96/56 for ACR/PASI). Overall, 136 patients were escalated to secukinumab 300 mg: 46 in secukinumab 150 mg load; 45 in secukinumab no-load; 45 in placebo-switchers