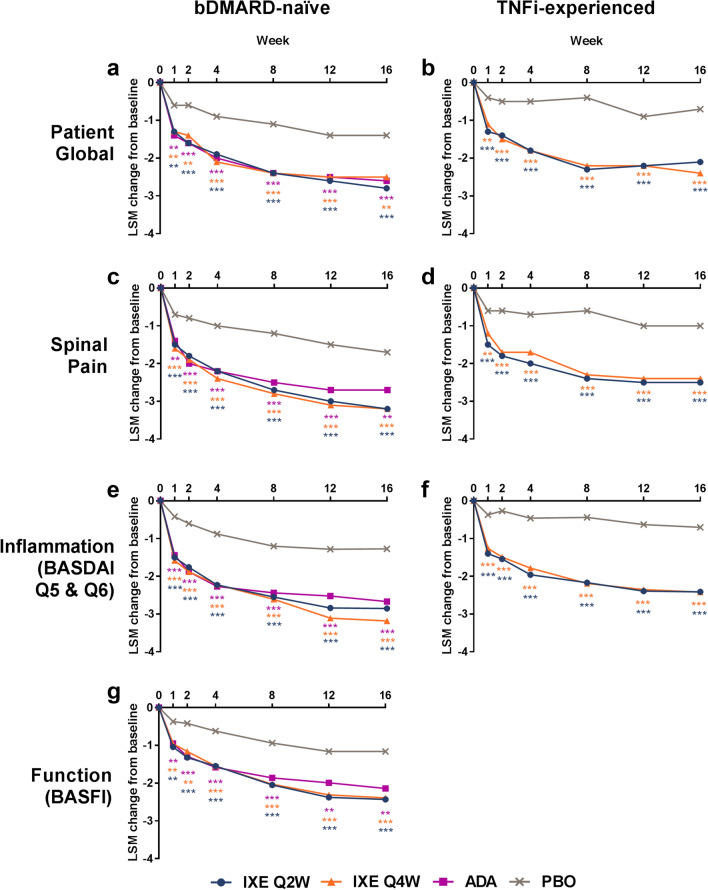
Fig. 1.
Least-squares mean (LSM) changes from baseline in the ASAS treatment response domains of a, b patient global, c, d spinal pain, e, f inflammation (BASDAI Q5 and Q6), and g function (BASFI) for a, c, e, g bDMARD-naïve patients (COAST-V) and b, d, f TNFi-experienced patients (COAST-W). Comparisons with PBO were made using a mixed-effects model for repeated measures. ***p < 0.001 vs. PBO; **p < 0.01 vs. PBO. The BASFI data for TNFi-experienced patients (COAST-W) were published previously [13]. ADA adalimumab, ASAS Assessment of Spondyloarthritis International Society, BASDAI Bath Ankylosing Spondylitis Disease Activity Index, BASFI Bath Ankylosing Spondylitis Functional Index, bDMARD biologic disease-modifying antirheumatic drugs, IXE ixekizumab, PBO placebo, Q2W, every 2 weeks, Q4W every 4 weeks, TNFi tumor necrosis factor inhibitor