Abstract
OBJECTIVES:
Previous reports have shown that high-frequency vibration can increase bone remodeling and accelerate tooth movement. The aim of this study was to evaluate the effects of high-frequency vibration on treatment phase tooth movement, and post-treatment bone density at initiation of retention, with cone-beam computed tomography (CBCT).
MATERIALS AND METHODS:
Thirty patients with initial Class I skeletal relationships, initial minimum-moderate crowding (3–5 mm), treated to completion with clear aligners and adjunctive high-frequency vibration, (HFV group) or no vibration, (Control group) were evaluated. The patients were instructed to change aligners as soon as they become loose. Changes in bone density associated with orthodontic treatment were evaluated using i-CAT cone-beam computed tomography (CBCT) and InVivo Anatomage® software to quantify density using Hounsfield units (HU) between treated teeth in 10 different regions. HU values were averaged and compared against baseline (T1) and between the groups at initiation of retention (T2).
RESULTS:
The average time for aligner change was 5.2 days in the HFV group, and 8.7 days in the control group (P = 0.0001). There was significant T1 to T2 increase of HU values in the upper arch (P = 0.0001) and the lower arch (P = 0.008) in the HFV group. There was no significant change in average HU values in the upper (P = 0.83) or lower arches (P = 0.33) in the control group. The intergroup comparison revealed a significant difference in the upper, (P = 0.0001) and lower arches (P = 0.007).
CONCLUSION:
High-frequency vibration adjunctive to clear aligners, allowed early aligner changes that led to shorter treatment time in minimum-moderate crowded cases. At initiation of retention, the HFV group demonstrated statistically significant increase as compared with pre-treatment bone density, whereas control subjects showed no significant change from pre-treatment bone density.
Keywords: Clear aligners, high-frequency vibration, orthodontic retention, tooth movement
Introduction and Background
Stability in retention is an important factor in the success of every completed orthodontic case.[1] In this new era, in which accelerated orthodontic treatment options are becoming standard offering, valid questions remain on the potential impacts of faster tooth movement on bone density entering the retention phase.
The literature presents conflicting reports on the effects of orthodontic tooth movement on bone density. Some studies have shown a reduction in bone density around orthodontically treated teeth,[2,3] while other studies have shown an increase in bone density.[4,5] Others still have reported no difference between baseline and final bone density.[6] A potential explanation may be different remodeling responses based on the type and magnitude of movement.[7]
The integrity of alveolar bone is dependent upon exposure to varying levels of mechanical stimulation,[8] Alveolar bone loss is associated with mechanical disuse such as excessive use of soft diet,[9] and missing teeth.[10] The sensitivity of alveolar bone to mechanical stimulation delivers the potential to augment both orthodontic tooth movement, and orthodontic retention with adjunctive vibratory mechanical stimulation.[11,12,13]
Vibration therapy is used in medicine to prevent, or minimize bone loss among adults with osteoporosis, children with muscular dystrophy and patients with cerebral palsy.[14,15,16] Vibration in the form of high-frequency 120 Hz, at 0.3 g acceleration, has recently shown to restore bone density to pre-disease levels among rats with ovariectomy induced osteoporotic alveolar bone.[17]
Research has shown that not all vibration is the same, and that bone cells are highly sensitive and responsive to changes in vibration frequency and magnitude.[18,19,20,21,22] Judex et al., subjected age-matched Sprague-Dawley rats to either 45 Hz or 90 Hz daily vibration and compared them to non-vibrated controls after 28 days. Bone formation among the lower frequency group (45 Hz), was not significantly different from controls, whereas the higher frequency group (90 Hz), demonstrated enhanced sensitivity and bone formation rates 159% greater than controls.[23]
For intraoral use, there are two types of vibrational devices available. Based on the aforementioned research devices operating at (≤45 Hz) will be referred to as low-frequency (LFV), and devices operating at (≥90 Hz) will be referred to as high-frequency (HFV). High-frequency vibration (120 Hz) has demonstrated to predictably produce either catabolic or anabolic changes within alveolar bone predicated on the presence, or absence of orthodontic force respectively.[12,18,24] This effect can be explained through significantly different environmental loading conditions on the periodontal ligament (PDL) and surrounding alveolar bone when in a state of constant pressure-tension, as compared to when in an unloaded or retention-state.[25,26]
The catabolic effect of 120 Hz HFV, adjunctive to orthodontic treatment, is based upon a synergistic increased inflammatory response of the PDL subjected to continuous compressive force. The enhanced catabolic cascade that follows HFV stimulation is responsible for the recruitment, differentiation, and significantly increased proliferation of osteoclast cells crucial for tooth movement.[11,12,26] This conclusion is supported by previous in-vitro and in-vivo experiments using vibration and compressive force on PDL and bone cells.[25,27,28,29]
The anabolic effect of HFV is based upon mechanosensory resident osteocytes embedded within the bone, making it sensitive and highly adaptive to mechanical stimulation.[15,17,18,30] This is achieved through a process called mechanotransduction in which mechanical signals are transduced into osteocyte bone cells and converted into biochemical energy.[31] Sclerostin produced by the Sost gene found within the osteocyte cell is a potent load-based regulator of bone density levels. In response to mechanical stimulation, osteocytes regulate sclerostin level output, inhibiting Wnt signaling to finely tune local and regional osteogenesis.[17,32]
In recent years, cone-beam computed tomographic (CBCT) imaging has been used instead of the multi-slice computed tomography in dentistry for diagnosis and treatment planning different dental and orthodontic procedures, as well as for evaluating mineralized tissues.[33,34] CBCT can provide adequate image quality with a lower radiation exposure dose compared to medical computed tomographic radiography (mCT). CBCT is faster and less invasive than biopsy, provides significant diagnostic advantages compared to two-dimensional images, and has become the specialists’ tool of choice to assess bone quality prior to implant surgery.[35,36,37] Alveolar bone density can be measured using Hounsfield units (HU) as derived from CBCT.[2,6,33,35,36,37] The Hounsfield unit (HU) scale is a linear transformation of the original linear attenuation coefficient measurement into one in which the radiodensity of distilled water at standard temperature and pressure (STP) is defined as zero Hounsfield units (HU), while the radiodensity of air at STP is defined as -1000 HU. With recent advances in hardware and software, accurate quantitative bone mineral density (BMD) measurements in Hounsfield units can be obtained from CBCT gray values through a calibration curve as described in the 2017 American Academy of Periodontology systematic review.[38] It is important to note that HU values obtained from CBCT do not represent absolute HU values as derived from Medical CT. The values do however provide clinicians valuable information relative to changes in bone density for comparative purposes within, and between patients.[36] Comparative accuracy is dependent upon the use of the same CBCT hardware, analysis software, and imaging process.[35,38]
Pre-clinical research of vibration adjunctive to orthodontic tooth movement has demonstrated the potential to change the chemistry, and quantity of alveolar bone around the moving tooth.[12] In their research Alikhani et al., identified the target cells, mechanism of action and demonstrated a significant acceleration of tooth movement at 120 Hz HFV compared to lower frequencies.[12] A recent human study with a similarly calibrated 120 Hz high-frequency device confirmed similar patterns of inflammatory cytokine upregulation which again correlated with increased rate of movement, and was accompanied by decreased reported orthodontic pain levels.[11] This trial included subjects using the same aligner system (Invisalign Smarttrack, Align technology, San Jose CA), the same aligner exchange interval (7-days). Notably, the variable associated with the significant change in biology was randomization to 5 minutes daily use of a bite-wafer type mouthpiece attached to either a sham or active HFV device (VPro, Propel Orthodontics, Ossining NY).
An unavoidable consequence of orthodontic treatment is the potential for root resorption. It's etiology is complex in nature, and inter-related to a multitude of factors including genetics, force levels, treatment duration, and bone density.[39,40] Root resorption and treatment time have been shown to be minimized when accompanied by HFV adjunctive to clear aligner orthodontic therapy.[41,42] The objective of this study was to further evaluate the effects of 120 Hz high-frequency vibration on the rate of tooth movement/aligner progression during active orthodontic treatment, and on post-treatment bone density at the initiation of the retention phase as evaluated from CBCT.
Hypotheses
The null hypotheses were (1) There is no significant difference in the rate of tooth movement/aligner progression between the HFV and control groups. (2) There is no significant difference in bone density changes after orthodontic treatment between the HFV and control group. The alternate hypothesis was that the HFV treated group would demonstrate accelerated tooth movement and improved bone density from baseline, as compared to the control group. This alternate hypothesis was based on the previously reported literature that adjunctive high-frequency mechanical vibration can accelerate bone turnover and remodeling.
Materials and Methods
This retrospective study included 30 consecutively treated patients that were treated with clear aligners (19 female/11 male). The criteria of patients’ inclusion were; Class I malocclusion at the beginning/before orthodontic treatment, good oral hygiene, complete permanent dentition, except third molars and initial anterior crowding ranging from ≥3 mm to ≤5 mm. Patients with any signs of alveolar bone defects such as; dehiscence, fenestration or other alveolar bone atrophic lesions observed at the pre-operative radiographic examination were not eligible for inclusion. Patients requiring any refinement after the end of their treatment were not eligible for inclusion. Informed consent was signed by all parents and/or guardians to use their radiographs for research purpose. This study has been approved by the ethical committee at the University of Alberta. All patients were treated by Invisalign SmartTrack® clear aligners programmed at default aligner rate of tooth movement of 0.25 mm maximum per aligner. This study was conducted in two different sites. Fifteen patients (mean age 24 ± 10 years) (mean # aligners 26) were trained to use a high-frequency vibration (HFV) device (VPro+, Propel Orthodontics, NY) designed to deliver a cyclical (vibrational) force with a frequency of 120 Hz for 5 minutes per day (HFV group). Control subjects (mean age 28 ± 11 years) (mean aligners 29) did not receive adjunctive vibration treatment, and instead were provided Chewies to serve as sham for aligner seating, (Control group). The patients in both sites were trained on how to seat their Invisalign aligners and how to identify proper vs problematic aligner tracking. They were instructed to wear the aligners 20-22 hours per day and to advance aligners as soon as they can comfortably insert the next sequential aligner with no gaps (saliva buildup, or bubbles) between the incisal/occlusal/facial edges of the teeth and the new aligner. If after 14 days their aligner is still not fitting properly, and they cannot advance to their aligner, they were instructed to immediately contact the treating office where alternative mitigation would be implemented such as back-tracking, decreasing tray interval, or mid-course correction, and therefore would not be eligible for inclusion in the study.
Cone beam computed tomography (CBCT) scans (iCAT®- Imaging Sciences International, Hatfield, Pennsylvania, USA) were taken for all patients before (T1) and after (T2) orthodontic treatment. The specifications of the CBCT are: 16 cm width × 13 cm height, 120 kVp, 24 mAs, 20 seconds’ scan time, 0.3 mm voxel size, and 303 basis projections. All subjects were provided with a protective lead apron.
Bone density measurements
For the pre and post-treatment CBCTs, InVivo Dental 5.0 (Anatomage Inc., San Jose, CA, USA, window width, 5000 HU; window level, 1500 HU), was used to measure bone density in the alveolar bone around the teeth in Hounsfield units (HU). To standardize the sections that were to be used for bone density analysis, the sagittal view of each patient's CBCT was obtained through InVivo Dental 5.0 software. The image was rotated so that the posterior rim of the incisive foramen and the posterior nasal spine were on the same slice [Figure 1]. In the upper arch, the coronal section was set at the level of the posterior rim of the incisive foramen [Figures 2a and b]. In the lower arch, the coronal section was set at the level midway between the point B and infradentale [Figures 3a and b]. In the standardized coronal slices of both arches, the bone density was measured midway between the buccal and lingual cortical plate in the cancellous bone of five different locations; bilaterally between canine and lateral incisor, bilaterally between lateral and central incisor, and at the midline [Figures 4a and b].
Figure 1.
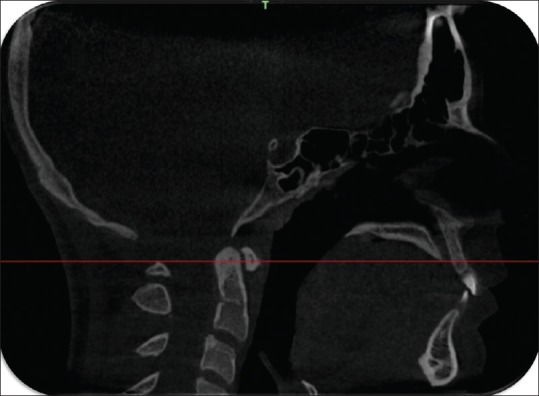
Sagittal section through the palate showing posterior rim of the incisive foramen and the posterior nasal spine were on the same slice
Figure 2.
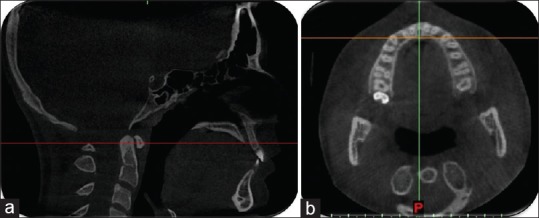
Reference lines for measuring the maxillary alveolar bone density passing through the posterior rim of the incisive foramen (a). The corresponding axial view of the maxilla (b)
Figure 3.
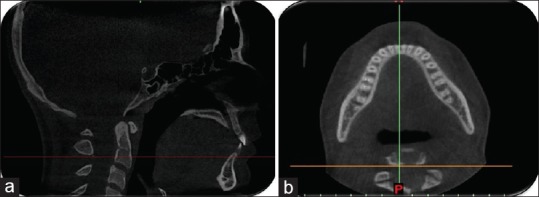
Reference lines for measuring the mandibular alveolar bone density passing through the posterior inferior point of the second cervical vertebra (a). The corresponding axial view of the mandible (b)
Figure 4.
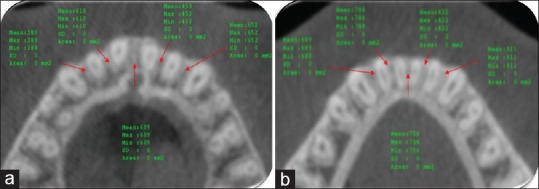
Measuring the bone density at five different locations; between the right canine and lateral, right lateral and central, left central and lateral, left lateral and canine and at the midline. HFV (a), Control (b)
Reliability test
Ten of the CBCT scans were randomly selected, and bone density measurements were repeated twice at fifteen days interval. The measurements were repeated by the same investigator to measure intra-operator reliability. Cronbach alpha (0.86) was calculated to assess the consistency between the two sets of bone density measurements. The value obtained indicates very good reliability of the measurements.
Statistical analysis
Statistical analysis was performed with SPSS Version 21.0 (SPSS Inc, Chicago, III). Shapiro-Wilk test was used to verify normal distribution of the data. Paired t-test was performed to evaluate bone density change within each group. Two sample t-test were performed for intergroup comparisons between variables.
Results
Aligner change/treatment time
The results of this study demonstrated that patients who received adjunctive high-frequency vibration exchanged clear aligners faster and finished their orthodontic treatment faster compared to controls. The HFV group average aligner exchange interval to comfortably advance aligners was 5.2 ± 2.2 days, whereas the control group average aligner exchange interval to comfortably advance aligners was 8.7 ± 1.2 days (P = 0.0001). The HFV group average total treatment time was 135 ± 27 days, whereas the control group average total treatment time was 252 ± 59 days (P = 0.002) [Table 1 and Figures 5, 6].
Table 1.
Aligner count, aligner interval, days in treatment and P value for Control and HFV groups
| Variable | Control | HFV | P |
|---|---|---|---|
| Aligner Count (Mean) | 29 | 26 | 0.22 |
| Aligner Interval (Mean) | 8.7±1.2 | 5.2±2.2 | 0.0001* |
| Treatment time in days (Mean) | 252±59 | 135±27 | 0.002* |
*Significant level (P<0.05)
Figure 5.
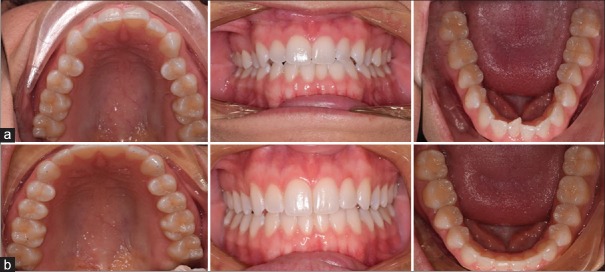
A patient from the HFV group with class I malocclusion (a) before and (b) after treatment with Invisalign clear aligners. Total treatment with 34 aligners was 175 days. (Average aligner change of 5.14 days)
Figure 6.
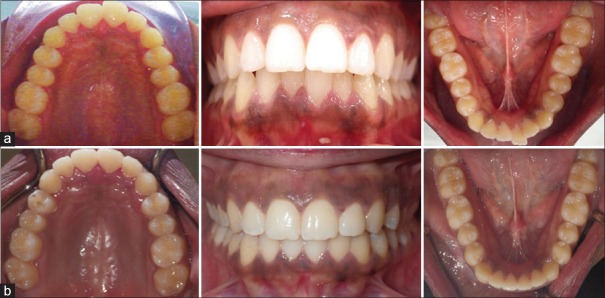
A patient from the control group with class I malocclusion (a) before and (b) after treatment with Invisalign clear aligners. Total treatment with 23 aligners was 203 days. (Average aligner change of 8.83 days)
Bone Density (intragroup comparison)
Alveolar Bone density as indicated by HU values difference of the HFV group increased significantly in both arches; (302.7 ± 35.1 HU value, P = 0.001) for the upper arch, and (101.4 ± 129.4 HU value, P = 0.008) for the lower arch [Table 2 and Figures 7a, b, 8 and 9]. Alveolar bone density of the control group did not show statistically significant differences between before and after HU values in either the upper (-0.44 ± 6.23 HU value, P = 0.83) or lower arches (2.86 ± 8.43 HU value, P = 0.33) [Table 3 and Figures 8, 9].
Table 2.
Paired t-test between the average pre-and post-treatment bone density as shown in the CBCT of the HFV group
| Variable | Pre-treatment T1 (HU) | Post-treatment T2 (HU) | P | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Max | Min | Mean | SD | Max | Min | ||
| Upper arch | 515.6 | 81.6 | 627.9 | 414.7 | 818.3 | 64.2 | 887.6 | 724 | 0.0001* |
| Lower arch | 563.1 | 101.9 | 811.6 | 401.8 | 664.2 | 91 | 785.2 | 558.4 | 0.008* |
*Significant level (P<0.05)
Figure 7.
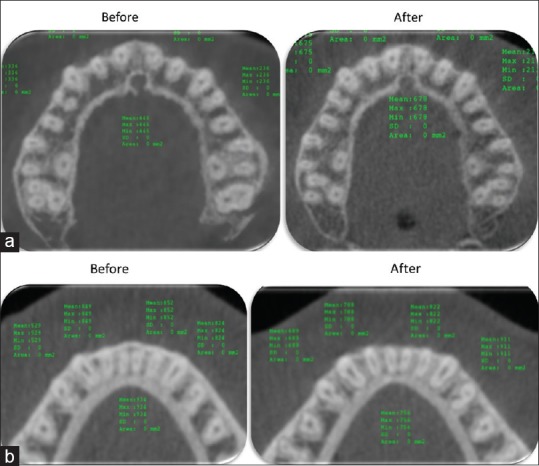
Bone density of the alveolar bone before and after HFV application, Upper arch (a) lower arch (b)
Figure 8.
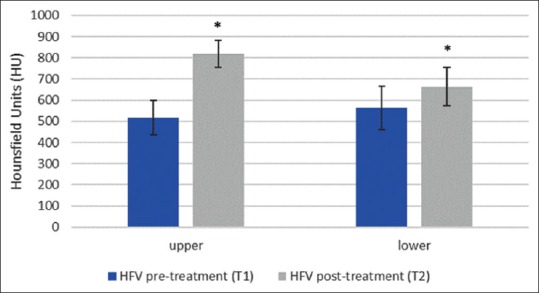
Graph showing the average pre-and post-treatment bone density changes in HU value in the HFV group. (*= statistically significant P < 0.05)
Figure 9.
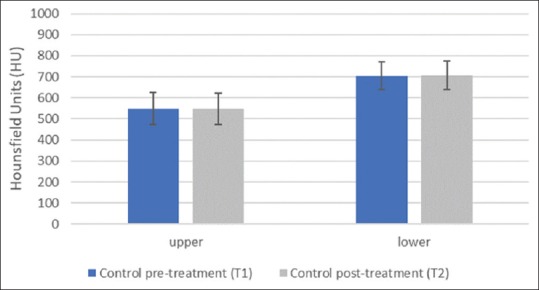
Graph showing the average pre-and post-treatment bone density changes in HU value in the Control group. (*= statistically significant P < 0.05)
Table 3.
Paired t-test between the average pre-and post-treatment bone density as shown in the CBCT of the Control group
| Variable | Pre-treatment T1 (HU) | Post-treatment T2 (HU) | P | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Max | Min | Mean | SD | Max | Min | ||
| Upper arch | 548.4 | 75.9 | 657.6 | 446.8 | 547.9 | 74.2 | 646 | 453 | 0.83 |
| Lower arch | 705.4 | 65.8 | 834 | 612 | 708.2 | 67.7 | 846.6 | 618.6 | 0.33 |
*Significant level (P<0.05)
Bone Density (intergroup comparison)
The overall comparison of the treatment changes between the two groups revealed a statistically significant difference in both the upper (P = 0.0001) and lower (P = 0.007) arches [Table 4].
Table 4.
Independent sample t-test for the bone density changes as shown in CBCT between the Control and HFV group
| Variable | Control group T2-T1 | HFV group T2-T1 | P | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Max | Min | Mean | SD | Max | Min | ||
| Upper arch | -0.44 | 6.23 | 7.6 | -11.6 | 302.7 | 35.1 | 259.7 | 309.3 | 0.0001* |
| Lower arch | 2.86 | 8.43 | 12.6 | -11.4 | 101.1 | 129.4 | -26.4 | 156.6 | 0.007* |
*Significant level (P<0.05)
Discussion
It has been shown that patients and clinicians are interested in methods to reduce orthodontic treatment time and are willing to pay a premium for it.[43] Adjunctive high-frequency vibration has demonstrated significant acceleration of tooth movement and has been used effectively with accelerated aligner exchange intervals.[11,12,28,41] Valid questions remain on the impacts of accelerated bone turnover on post treatment bone density entering the retention phase.
This study evaluated the effect of adjunctive high-frequency vibration (HFV) on orthodontic treatment time and post-treatment bone density at initiation of retention phase, using clear aligners. The results demonstrated subjects using high-frequency vibration were able to advance aligners 40% faster than controls while demonstrating a statistically significant net increase in bone density as compared to baseline at the completion of orthodontic treatment. Control subjects demonstrated no statistical difference between beginning and final bone density. To our best knowledge, this is the first study to investigate the effect of high-frequency mechanical vibration on bone density after orthodontic treatment in humans using the CBCT.
Aligner therapy continues to advance in technology and clinical utilization. Aligner protocols aimed at maximizing treatment efficiency while improving patient experience are being redefined and refined. In the present study, all patients were instructed to change aligners as soon as they became loose or passive, allowing a comfortable advance to the next aligner. Three possible explanations for the faster rate of aligner change in the HFV group could be due to one or all of the following. First, 5 minutes per day use of the device may provide better mechanics through consistent aligner seating throughout the dentition, and subsequently more consistent force delivery throughout treatment duration.[44,45] Second, it is well recognized that high-frequency vibration can provide pain relief from dental origin, and therefore patients may have experienced greater compliance with their aligners as well as with the 5-minute HFV treatment per day.[11,46] Thirdly, the rate-limiting biological response may have been directly amplified by the HFV device,[11,12,19,22,28,47] resulting in accelerated bone remodeling, and a corresponding faster rate of tooth movement.
Craniofacial bones are reponsive to mechanical stimulation.[8,9] In the absence of orthodontically induced inflammation, vibration demonstrates strong anabolic effects. Alikhani systematically investigated vibration in the absence of orthodontic force, at 30 Hz, 60 Hz, 100 Hz, and 200 Hz vs non-vibrated controls.[18] Osteoblast production significantly increased for all frequencies with alveolar bone volume increases of 10%, 17%, 19%, and 12%, respectively, compared with untreated animals. On the cellular level, this increased anabolic effect of mechanical stimulation can be attributed to strain inhibition of RANKL induced by bone stromal cells.[48,49]
Osteoclast proliferation delivers localized reductions in bone density necessary for tooth movement, and has been shown to be upregulated via PDL cells in response to a static compressive force.[12,27] Yamamoto demonstrated high-frequency vibration to enhance osteoclast proliferation versus non-vibrated controls only when applied adjunctive to a continuous static force.[28] Leethanakul, et al., 2016 demonstrated in a randomized, controlled, split-mouth trial that high-frequency vibration (125 Hz) adjunctive to orthodontic force accelerated tooth movement and was accompanied by increased levels of cytokine IL-1b.[47] Cytokine IL-1b is involved in osteoclast activation, differentiation and survival which supports accelerated tooth movement.[50,51] In their trial, HFV was investigated adjunctive to fixed appliance treatment, and experimental subjects demonstrated a 62% faster rate of tooth movement versus controls. Alikhani, et al., systematically investigated vibration in the presence of orthodontic force, (aseptic inflammatory condition), at 30 Hz, 60 Hz, and 120 Hz vs non-vibrated controls.[12] Results demonstrated a direct correlation between frequency and tooth movement. As frequency increased, tooth movement increased with 120 Hz producing the greatest magnitude of tooth movement, statistically significant compared to both control and 30 Hz subjects. Acceleration g-force was simultaneously investigated and shown to be a critical component to a catabolic vibration profile. 120 Hz at 0.01 g did not trigger the catabolic cascade and accelerate tooth movement, whereas 120 Hz at 0.05 g did become catabolic increasing cytokines, osteoclasts and significantly accelerated tooth movement. Therefore, the increase in tooth movement not only correlated with frequency, it depended upon a clinically relevant acceleration g-force from the vibration device to increase cytokines and subsequent osteoclast proliferation. Further, concomitant NSAID application was able to block this catabolic effect.[12] This demonstrates vibrations impact on tooth movement to be frequency sensitive, acceleration (g-force) dependent and only occur in the presence of an inflammatory baseline condition, such as is present during the application of orthodontic forces.
These findings help toward answering questions surrounding conflicting reports among studies, and significantly advance our understanding of vibration applications for use in dentistry. If adjunctive vibration is not triggering a catabolic cascade, the mechanical stimulation delivered appears only anabolic in nature which could be counter-productive to accelerated orthodontic tooth movement.
Considering final aligners are often staged to overcorrect a simple motion such as a single rotation, or can be entirely passive, high-frequency vibration would therefore have ceased PDL amplification of the osteoclastic effect on non-activated teeth, and instead through increased mechanical stimulation only enhance the osteoblastic bone deposition phase.[17,18,28] This theory is in agreement with Alikhani and Yamamoto's findings of HFV's increased catabolic effect in the presence of force,[12,28] and Alikhani's findings of that HFV's increased anabolic effect, in the absence of orthodontic force.[17,18] This anabolic effect was also shown to be frequency correlated, and as frequency increased from 30 Hz to 100 Hz the osteogenic effect nearly doubled.
There are important limitations to be considered when interpreting the results of this study. First, while many studies have previously demonstrated the CBCT gray values are routinely used and clinically suitable for assessing bone density, other studies have indicated they do not accurately assess bone density. The CBCT methodology used in this research was approved by the University of Alberta health ethics review board (HERB). It was based on the American Dental Association (ADA) position statement,[52] and the American Academy of Oral and Maxillofacial Radiology (AAOMR) clinical recommendations,[53] for achieving clinically relevant images while adhering to the “as low as reasonably achievable” (ALARA) principle, and comparable to many published reports quantitatively measuring bone density.[2,6,33,35,36,37] Second, this research measured the alveolar bone density only in the anterior region. This was based on the inclusion criteria of correcting class I anterior crowding. Changes in bone density around the teeth in the posterior region also merits investigation. Third, patients were instructed to exchange aligners once passive. While this may seem to impart variability, it is biologically most efficient and is by design aimed at reducing variability in stage completion that can result in poor tracking. Not allowing the advancement of aligners after individually staged movements are completed is equivalent to prescribing intermittent forces. Intermittent forces have been clinically proven less effective than continuous forces.[54] Mandating aligner advancement on a strict, fixed, but arbitrary schedule may actually impart increased variability based on whether or not the sequentially staged forces had previously become passive or were not yet completed delivering incomplete and elevated forces. This limitation is inherent with sequential aligners and unavoidable.
In the current study, patients treated with HFV demonstrated faster aligner changes, and significantly improved post-treatment, retention phase, alveolar bone density. The results do not fully support the null hypotheses; Hypothesis 1 is rejected. The HFV group demonstrated faster rate of tooth movement/aligner change when compared to control. Hypothesis 2 is rejected. The HFV group demonstrated significant change between T1 and T2 bone density when compared to control. This study demonstrates the potential benefit of HFV in accelerating treatment, while improving bone density for retention. Post-orthodontic bone density is important to support occlusal stability and prevent orthodontic relapse.[55] These findings may support the potential for HFV to improve or even accelerate bone mineralization earlier in the orthodontic retention phase. Future research on the use of high-frequency vibration in the retention phase is warranted to evaluate its potential impact on decreasing the time required for full-time orthodontic retention prior to night-time only wear and reducing orthodontic relapse.
The ease of use, and absence of any known side effects makes barriers to integration of the high-frequency device low. Other dental applications requiring enhancement of alveolar bone such as to increase the stability of the prosthetic implants, preserve the integrity of the bone under dentures and periodontal disease management may also benefit from high-frequency mechanical stimulation.
Conclusions
The use of a 120Hz high-frequency vibration device during orthodontic treatment using clear aligners significantly accelerated aligner change and corresponding tooth movements
The use of a 120Hz high-frequency vibration device, significantly improved bone density from pre-treatment levels which could provide clinically meaningful benefits for pre-restorative set-ups, retention, and post-orthodontic stability.
List of abbreviations
AAOMR american academy of oral and maxillofacial radiology
ADA american dental association
ALARA as low as reasonably achievable
CBCT cone-beam computed tomography
HFV high-frequency vibration
HU hounsfield units
Hz hertz (cycles per second)
mCT medical computed tomographic radiography
NSAID non-steroidal anti-inflammatory drug
PDL periodontal ligament.
Declarations
Authors’ contributions
TE conceived of the study and participated in its design, coordination, and patient treatment. TS participated in the design of the study and patient treatment. KF participated in the design of the study and performed the statistical analysis. All authors read and approved the final manuscript. All authors confirm that the content of the manuscript has not been published or submitted for publication elsewhere.
Availability of data and material
The datasets supporting the conclusions of this article are included within the article.
Competing interests
TE and TS have held lectures for Align Technology in the past 5 years. TS has held lectures for Propel Orthodontics in the past 5 years.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All procedures in this study were approved by the Ethics Committee of the University of Alberta and complied with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Financial support and sponsorship
The present study University Research IRB application was funded by Propel Orthodontics LLC. No other compensation was provided.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
None to report.
References
- 1.Miles P, Rinchuse D. Orthodontic retention and stability: A clinical perspective. J Clin Ortho. 2007;41:125–32. [PubMed] [Google Scholar]
- 2.Ma Z, Yang C, Fang B, Xia Y, Mao L, Feng Y. Three-D imaging of dental alveolar bone change after fixed orthodontic treatment in patients with periodontitis. Int J Clin Exp Med. 2015;8:2385–91. [PMC free article] [PubMed] [Google Scholar]
- 3.Yu J, Huang H, Liu C, Wu J, Li Y, Tsai MT, et al. Does orthodontic treatment affect the alveolar bone density.? Medicine. 2016;95:e3080. doi: 10.1097/MD.0000000000003080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ru N, Liu S, Zhuang L, Li S, Bai Y. In vivo microcomputed tomography evaluation of rat alveolar bone and root resorption during orthodontic tooth movement. Angle Orthod. 2013;83:402–9. doi: 10.2319/031312-219.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhuang L, Bai, Y, Meng X. Three-dimensional morphology of root and alveolar trabecular bone during tooth movement using micro-computed tomography. Angle Orthod. 2011;81:420–5. doi: 10.2319/071910-418.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.da Silva Campos M, Gomes de Albuquerque E, Hauck Pinto B, Hungaru, H, Gravina M, et al. The role of orthodontic tooth movement in bone and root mineral density: A study of patients submitted and not submitted to orthodontic treatment. Med Sci Monit. 2012;18:CR752–7. doi: 10.12659/MSM.883604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verna C, Dalstra M, Melsen B. The rate and the type of orthodontic tooth movement is influenced by bone turnover in a rat model. Eur J Orthod. 2000;22:343–52. doi: 10.1093/ejo/22.4.343. [DOI] [PubMed] [Google Scholar]
- 8.Herring SW. Masticatory muscles and the skull: A comparative perspective. Arch Oral Biol. 2007;52:296–9. doi: 10.1016/j.archoralbio.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mavropoulos A, Kiliaridis S, Bresin A, Ammann P. Effect of different masticatory functional and mechanical demands on the structural adaptation of the mandibular alveolar bone in young growing rats. Bone. 2004;35:191–7. doi: 10.1016/j.bone.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Van der Weijden F, Dell’Acqua F, Slot DE. Alveolar bone dimensional changes of post-extraction sockets in humans: A systematic review. J Clin Periodontol. 2009;36:1048–58. doi: 10.1111/j.1600-051X.2009.01482.x. [DOI] [PubMed] [Google Scholar]
- 11.Alansari S, Atique M, Gomez J, Hamidaddin M, Thirumoorthy S, Sangsuwon C, et al. The effects of brief daily vibration on clear aligner orthodontic treatment. J World Fed Orthod. 2018:7134–40. [Google Scholar]
- 12.Alikhani M, Alansari S, Hamidaddin MA, Sangsuwon C, Alyami B, Thirumoorthy SN, et al. Vibration paradox in orthodontics: Anabolic and catabolic effects. PLoS One. 2018;13:e0196540. doi: 10.1371/journal.pone.0196540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teixera C, Nervina JM, Alansari S, Sangsuwon C, Alikhani M. High frequency acceleration: A new tool for alveolar bone regeneration. JSM Dent Surg. 2017;2:1026. [PMC free article] [PubMed] [Google Scholar]
- 14.Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K, et al. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: A clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res. 2004;19:343–51. doi: 10.1359/JBMR.0301251. [DOI] [PubMed] [Google Scholar]
- 15.Ward K, Alsop C, Caulton J, Rubin C, Adams J, Mughal Z. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J Bone Miner Res. 2004;19:360–9. doi: 10.1359/JBMR.040129. [DOI] [PubMed] [Google Scholar]
- 16.Gilsanz V, Wren TA, Sanchez M, Dorey F, Judex S, Rubin C. Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J Bone Miner Res. 2006;21:1464–74. doi: 10.1359/jbmr.060612. [DOI] [PubMed] [Google Scholar]
- 17.Alikhani M, Alikhani M, Alansari S, Almansour A, Hamidaddin MA, Khoo E, et al. Therapeutic effect of localized vibration on alveolar bone of osteoporotic rats. PLoS One. 2019;14:e0211004. doi: 10.1371/journal.pone.0211004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alikhani M, Khoo E, Alyami B, Raptis M, Salgueiro JM, Oliveira SM, et al. Osteogenic effect of high-frequency acceleration on alveolar bone. J Dent Res. 2012;91:413–9. doi: 10.1177/0022034512438590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Judex S, Pongkitwitoon S. Differential efficacy of 2 vibrating orthodontic devices to alter the cellular response in osteoblasts, fibroblasts, and osteoclasts. Dose Response. 2018;16:1559325818792112. doi: 10.1177/1559325818792112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagaraja M, Jo H. The role of mechanical stimulation in recovery of bone loss-high versus low magnitude and frequency of force. Life. 2014;4:117–30. doi: 10.3390/life4020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Judex S, Koh TJ, Xie L. Modulation of bone's sensitivity to low-intensity vibrations by acceleration magnitude, vibration duration, and number of bouts. Osteoporos Int. 2015;26:1417–28. doi: 10.1007/s00198-014-3018-5. [DOI] [PubMed] [Google Scholar]
- 22.Judex S, Rubin C. Is bone formation induced by high-frequency mechanical signals modulated by muscle activity? J Musculoskelet Neuronal Interact. 2010;10:3–11. [PMC free article] [PubMed] [Google Scholar]
- 23.Judex S, Lei X, Han D, Rubin C. Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J Biomechanics. 2007;40:1333–9. doi: 10.1016/j.jbiomech.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Alikhani M, Lopez J, Alabdullah H, Vongthongleur T, Sangsuwon C, Alikhani M, et al. High-frequency acceleration: Therapeutic tool to preserve bone following tooth extractions. J Dent Res. 2016;95:311–8. doi: 10.1177/0022034515621495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dutra E, Nanda R, Yadav S. Bone response of loaded periodontal ligament. Curr Osteoporos Rep. 2016;14:280. doi: 10.1007/s11914-016-0328-x. [DOI] [PubMed] [Google Scholar]
- 26.Krishnan V, Davidovitch Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofacial Orthop. 2006;129:e1–469.e32. doi: 10.1016/j.ajodo.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Kanzaki H, Chiba M, Shimizu Y, Mitani H. Periodontal ligament cells under mechanical stress induce osteoclastogenesis by receptor activator of nuclear factor B ligand up-regulation via prostaglandin E2 synthesis. J Bone Miner Res. 2002;17:210–20. doi: 10.1359/jbmr.2002.17.2.210. [DOI] [PubMed] [Google Scholar]
- 28.Takano-Yamamoto T, Sasaki K, Fatemeh G, Fukunaga T, Seiryu M, Daimaruya T, et al. Synergistic acceleration of experimental tooth movement by supplementary high-frequency vibration applied with a static force in rats. Sci Rep. 2017;7:13969. doi: 10.1038/s41598-017-13541-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leethanakul C, Suttapreyasri S, Pravitharangul A, Chatmahamongkol C. The effect of compressive force combined with mechanical vibration on human alveolar bone osteoblasts. J Oral Biol Craniofac Res. 2019;9:81–5. doi: 10.1016/j.jobcr.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubin C, Judex S, Garman R. Small oscillatory accelerations, independent of matrix deformations, increase osteoblast activity and enhance bone morphology. PLoS One. 2007;2:e653. doi: 10.1371/journal.pone.0000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liedert A, Kaspar D, Blakytny R, Claes L, Ignatius A. Signal transduction pathways involved in mechanotransduction in bone cells. Biochem Biophys Res Commun. 2006;349:1–5. doi: 10.1016/j.bbrc.2006.07.214. [DOI] [PubMed] [Google Scholar]
- 32.Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–75. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 33.Aranyarachkul P, Caruso J, Gantes B, Schulz E, Riggs M, Dus I, et al. Bone density assessments of dental implant sites: 2. Quantitative cone-beam computerized tomography. Int J Oral Maxillofac Implants. 2005;20:416–24. [PubMed] [Google Scholar]
- 34.Kapila SD, Nervina JM. CBCT in orthodontics: Assessment of treatment outcomes and indications for its use. Dentomaxillofac Radiol. 2015;44:20140282. doi: 10.1259/dmfr.20140282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mah P, Reeves TE, McDavid WD. Deriving Hounsfield units using grey levels in cone beam computed tomography. Dentomaxillofac Radiol. 2010;39:323–35. doi: 10.1259/dmfr/19603304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reeves TE, Mah P, McDavid WD. Deriving Hounsfield units using grey levels in cone beam CT: A clinical application. Dentomaxillofac Radiol. 2012;41:500–8. doi: 10.1259/dmfr/31640433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim D. Can dental cone beam computed tomography assess bone mineral density? J Bone Metab. 2014;21:117–26. doi: 10.11005/jbm.2014.21.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rios HF, Borgnakke WS, Benavides E. The use of cone-beam computed tomography in management of patients requiring dental implants: An American Academy of Periodontology Best Evidence Review. J Periodontol. 2017;88:946–59. doi: 10.1902/jop.2017.160548. [DOI] [PubMed] [Google Scholar]
- 39.Kocadereli I, Yesil TN, Veske PS, Uysal S. Apical root resorption: A prospective radiographic study of maxillary incisors. Eur J Dent. 2011;5:318–23. [PMC free article] [PubMed] [Google Scholar]
- 40.Ozkalaycia N, Karadenizb E, Elekdag-Turkc S, Turkd T, Chenge L, Darendeliler MA, et al. Effect of continuous versus intermittent orthodontic forces on root resorption: A microcomputed tomography study. Angle Orthod. 2018;88:733–9. doi: 10.2319/012518-68.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shipley T. Effects of high frequency acceleration device on aligner treatment—A pilot study. Dent J. 2018;6:32. doi: 10.3390/dj6030032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farouk K, Shipley T, El-Bialy T. Effect of the application of high-frequency mechanical vibration on tooth length concurrent with orthodontic treatment using clear aligners: A retrospective study. J Orthodont Sci. 2018;7:20. doi: 10.4103/jos.JOS_53_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uribe F, Padala S, Allareddy V, Nanda R. Patients’, parents’, and orthodontists’ perceptions of the need for and costs of additional procedures to reduce treatment time. Am J Orthod Dentofacial Orthop. 2014;145(Suppl):S65–73. doi: 10.1016/j.ajodo.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 44.Simon M, Keilig L, Schwarze J, Jung B, Bourauel C. Treatment outcome and efficacy of an aligner technique – regarding incisor torque, premolar derotation and molar distalization. BMC Oral Health. 2014;14:68. doi: 10.1186/1472-6831-14-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yezdani A. Transparent aligners: An invisible approach to correct mild skeletal class III malocclusion. J Pharm Bioallied Sci. 2015;7(Suppl 1):S301–6. doi: 10.4103/0975-7406.155965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ottoson D, Ekblom A, Hansson P. Vibratory stimulation for the relief of pain of dental origin. Pain. 1981;10:3745. doi: 10.1016/0304-3959(81)90043-9. [DOI] [PubMed] [Google Scholar]
- 47.Leethanakul C, Suamphan S, Jitpukdeebodintra S, Thongudomporn U, Charoemratrote C. Vibratory stimulation increases interleukin-1 beta secretion during orthodontic tooth movement. Angle Orthod. 2016;86:74–80. doi: 10.2319/111914-830.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rubin J, Murphy TC, Fan X, Goldschmidt M, Taylor WR. Activation of extracellular signal-regulated kinase is involved in mechanical strain inhibition of RANKL expression in bone stromal cells. J Bone Miner Res. 2002;17:1452–60. doi: 10.1359/jbmr.2002.17.8.1452. [DOI] [PubMed] [Google Scholar]
- 49.Xie L, Jacobson JM, Choi ES, Busa B, Donahue LR, Miller LM, et al. Low-level mechanical vibrations can influence bone resorption and bone formation in the growing skeleton. Bone. 2006;39:1059–66. doi: 10.1016/j.bone.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 50.Kim J, Jin H, Kim K, Song I, Youn BMatsuo K, Kim N. The Mechanism of Osteoclast Differentiation Induced by IL-1. J Immunol. 2009;183:1862–70. doi: 10.4049/jimmunol.0803007. [DOI] [PubMed] [Google Scholar]
- 51.Lee Y, Fujikado N, Manaka H, Yasuda H, Iwakura Y. IL-1 plays an important role in the bone metabolism under physiological conditions. Int Immunol. 2010;22:805–16. doi: 10.1093/intimm/dxq431. [DOI] [PubMed] [Google Scholar]
- 52.American Dental Association Council on Scientific Affairs. The use of cone-beam computed tomography in dentistry: An advisory statement from the American Dental Association Council on Scientific Affairs. J Am Dent Assoc. 2012;143:899–902. doi: 10.14219/jada.archive.2012.0295. [DOI] [PubMed] [Google Scholar]
- 53.American Academy of Oral and Maxillofacial Radiology. Clinical recommendations regarding use of cone beam computed tomography in orthodontics. [corrected]. Position statement by the American Academy of Oral and Maxillofacial Radiology. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:238–57. doi: 10.1016/j.oooo.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 54.Buschang P, Kyung H, Liu S. Continuous forces are more effective than intermittent forces in expanding sutures. Eur J Orthod. 2010;32:371–80. doi: 10.1093/ejo/cjp103. [DOI] [PubMed] [Google Scholar]
- 55.Nicholson PT, Plint DA. A long-term study of rapid maxillary expansion and bone grafting in cleft lip and palate patients. Eur J Orthod. 1989;11:186–92. doi: 10.1093/oxfordjournals.ejo.a035982. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article.