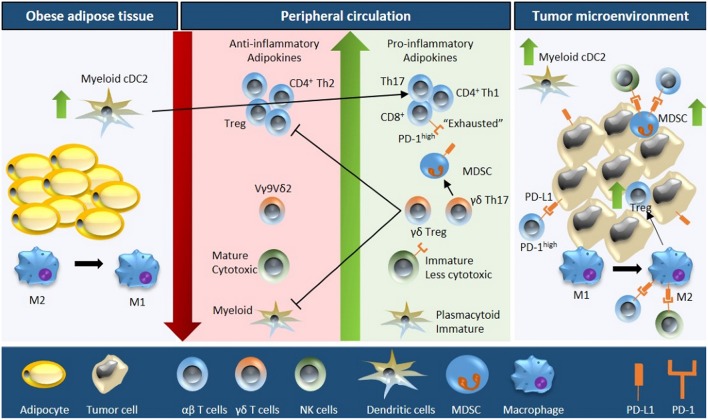
Figure 1.
Schematic overview of obesity-associated immune modulations in cancer. Fat accumulation in adipocytes triggers a pro-inflammatory microenvironment within the adipose tissue characterized by M2 to M1 macrophage polarization and accumulation of myeloid conventional DC2 (cDC2) cells. Obese adipose tissue increases the secretion of pro-inflammatory adipokines and free fatty acids concomitant with a downregulation of anti-inflammatory adipokines into the peripheral circulation. As a consequence, the number and activity of cytotoxic T cells decreases (due to reduced proliferation, increased apoptosis, and impaired function of the progenitor thymocytes), NK cell maturation is defective, the plasmacytoid to myeloid DC ratio increases, the number of MDSCs is upregulated, and Vγ9Vδ2 cells are polarized into γδ Treg and γδ T17 cells (further inhibiting the function of cytotoxic T cells and myeloid DCs). Moreover, the obese microenvironment increases the expression of PD-1 on T cells and NK cells, and of PD-L1 on MDSCs. These systemic alterations ultimately result in increased immune evasion, especially due to the interaction of PD-1/PD-L1, increased tumor MDSC and Treg infiltration, and M1 to M2 macrophage polarization; resulting in an immunosuppressive tumor microenvironment.