Figure 1.
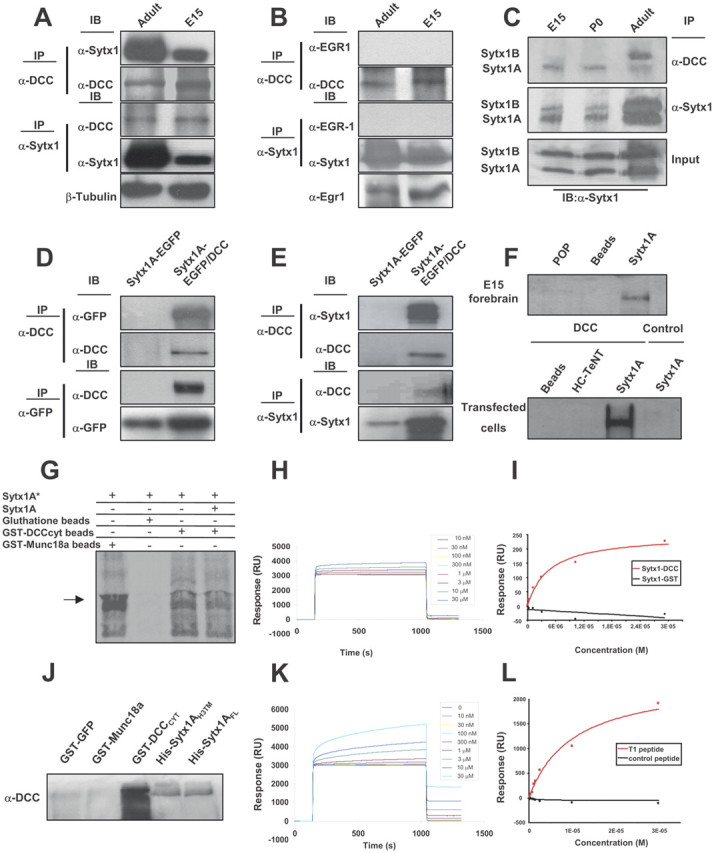
Sytx1 coassociates with the DCC receptor in brain tissue and in transfected cells. A, DCC and Sytx1 immunoprecipitation of E15 and adult forebrain homogenates. DCC immunoprecipitation yields coimmunoprecipitation of Sytx1 in both samples (top panels). Sytx1 immunoprecipitation yields coassociation of DCC (bottom panels). Anti-βIII-tubulin antibodies were used as loading controls. B, DCC and Sytx1 immunoprecipitation assays do not result in coimmunoprecipitation of Egr1. Levels of Egr1 protein lysates are shown (bottom). C, DCC associates with A and B isoforms of Sytx1. Forebrain homogenates were immunoprecipitated and immunocomplexes were subjected to urea/SDS-PAGE. DCC coimmunoprecipitates two Sytx1 bands corresponding to Sytx1A and 1B (top panel). D, E, Coimmunoprecipitation experiments in HEK293 cells. DCC immunoprecipitation results in coassociation with Sytx1A, visualized either with anti-GFP (D) or anti-Sytx1 (E) antibodies. The reverse immunoprecipitation with anti-GFP or anti-Sytx1 antibodies also reveals DCC (D, E). Note that the efficiency of the immunoprecipitation with anti-GFP antibodies is consistently higher than when using anti-DCC antibodies, which yields low recovery of proteins in this condition. F, DCC affinity pull-down experiments with purified His-Sytx1A. Incubation with anti-DCC antibody reveals DCC in the beads coupled to His-Sytx1A, but not in control beads. G, Sytx1A was transcribed and translated in vitro in the presence of [35S]Met, and incubated with glutathione-Sepharose beads coupled to GST-DCCCYT or GST-MUNC18a. After SDS-PAGE, gels were exposed to a storage phosphor screen. [35S]Met-Sytx1A binds to DCCCYT (as well as to MUNC18a), but not to empty beads. Binding of [35S]Met-Sytx1A to DCCCYT is decreased in the presence of nonradioactive Sytx1A (Sytx1A*). H, Sensorgram showing binding between the GST-DCCCYT domain and His-Sytx1A. Increasing concentrations of His-Sytx1A were injected into a chip where the GST-DCCCYT was cross-linked; the interaction was recorded as SPR changes [in response units (RU)]. Increasing concentrations of His-Sytx1A yielded higher responses. I, Plot of the steady-state response (in RU) between GST-DCCCYT and a range of concentrations of His-Sytx1A (red) or between control GST and His-Sytx1A (black). No binding is detected when GST protein was cross-linked, whereas a saturable response is observed when GST-DCCCYT was immobilized. J, Pull-down experiments in which brain extracts (P0) were passed through Ni2+-affinity columns to which recombinant His-Sytx1A or His-Sytx1AH3TM proteins were coupled. Western blot analyses show coprecipitation of DCC with similar efficiencies in both cases. No coprecipitation of DCC is detected when extracts are incubated with control GST-GFP or GST-MUNC18a columns, whereas strong coprecipitation was observed with GST-DCCcyt. The high DCC signal in GST-DCCcyt samples is probably due to multimerization of the DCC receptor through the P3 domain. K, Sensorgram showing binding between the T1DCC peptide and purified His-Sytx1A. The peptide was immobilized as described in Material and Methods, and then increasing concentrations of His-Sytx1A were injected. Responses (in RU) increase at increasing concentrations of His-Sytx1A as a function of the time (in seconds). L, Plot of the steady-state response between T1 peptide and His-Sytx1A (red) or between a control peptide and His-Sytx1A (black). While no binding is detected when the control peptide is cross-linked, specific binding is observed when T1 peptide is immobilized.
