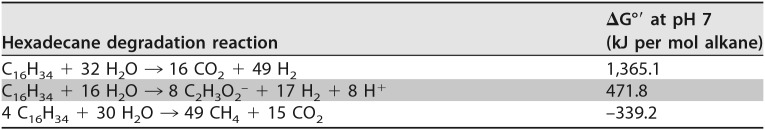
TABLE 2.
Thermodynamic calculations for the degradation of hexadecane under different conditionsa
| Hexadecane degradation reaction | ΔG°′ at pH 7 (kJ per mol alkane) |
|---|---|
| C16H34 + 32 H2O → 16 CO2 + 49 H2 | 1,365.1 |
| C16H34 + 16 H2O → 8 C2H3O2– + 17 H2 + 8 H+ | 471.8 |
| 4 C16H34 + 30 H2O → 49 CH4 + 15 CO2 | –339.2 |
Complete oxidation, oxidation to acetate, and oxidation coupled with methane formation.