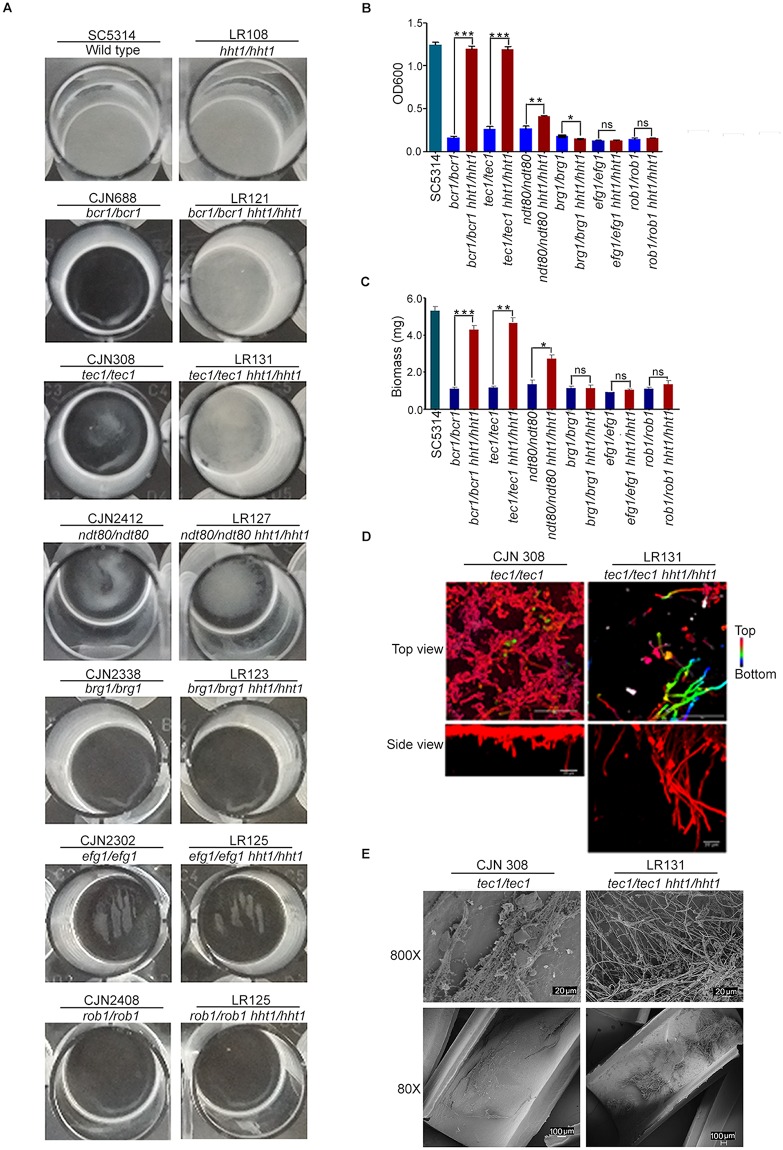
Fig 5. Deletion of the variant histone H3 rescues defects in biofilm formation associated with mutants of 3 biofilm master regulators.
(A) In vitro biofilm formation assay was performed using single mutants of each of the 6 master regulators of biofilm formation, (Bcr1, Tec1, Ndt80, Brg1, Efg1, and Rob1) and the corresponding double-mutant strains in which H3VCTG was deleted in each mutant background. Biofilms were grown in YPD medium in 24-well polystyrene plates for 24 hours at 37 °C. Biofilm formation defects were significantly rescued in bcr1, tec1, and ndt80 null mutants in the absence of H3VCTG. (B) Biofilm formation was quantified using the Standard Optical Density Assay by measuring OD600 of the cells adhered to the bottom of the plates. In vitro biofilm assay was performed in YPD using indicated single- and corresponding double-mutant strains. Biofilms were grown in 24-well plates for 24 hours at 37 °C. Data are the mean of 3 independent wells per condition. Error bars represent the standard deviation. The data underlying this figure can be found in S2 Data. (C) Biomass of the wild type and each single- and double-mutant strains grown in YPD in 24-well plates for 24 hours at 37 °C. The data underlying this figure can be found in S2 Data. (D) tec1 single mutant and tec1 hht1 double mutant were adhered to silicone squares in a 12-well polystyrene plate in YPD at 37 °C, and biofilms were allowed to form for 48 hours at 37 °C. Biofilms were stained with concanavalin A-Alexa Fluor 594 and imaged by CLSM. Images represent projections of the top and side views. Representative images of at least 3 replicates are shown. Scale bars: 50 μm. (E) Biofilm assay was performed in vivo by using a rat catheter model. CJN308 (tec1/tec1) or LR131 (tec1/tec1 hht1/hht1) were inoculated in the rat intravenous catheter and were allowed to form a biofilm. After 24 hours of incubation, biofilms were visualized using SEM. The images are 80× and 800× magnification views of the catheter lumens. Bcr1, biofilm and cell wall regulator 1; Brg1, biofilm regulator 1; CLSM, confocal laser scanning microscopy; Efg1, enhanced filamentous growth protein 1; Ndt80, non-dityrosine 80; OD, optical density; Rob1, regulator of biofilm 1; SEM, scanning electron microscopy; Tec1, transposon enhancement control 1; YPD, yeast peptone dextrose.