ABSTRACT
Tendon and bone are attached by a transitional connective tissue that is morphologically graded from tendinous to osseous and develops from bipotent progenitors that co-express scleraxis (Scx) and Sox9 (Scx+/Sox9+). Scx+/Sox9+ progenitors have the potential to differentiate into either tenocytes or chondrocytes, yet the developmental mechanism that spatially resolves their bipotency at the tendon-bone interface during embryogenesis remains unknown. Here, we demonstrate that development of Scx+/Sox9+ progenitors within the mammalian lower jaw requires FGF signaling. We find that loss of Fgfr2 in the mouse tendon-bone interface reduces Scx expression in Scx+/Sox9+ progenitors and induces their biased differentiation into Sox9+ chondrocytes. This expansion of Sox9+ chondrocytes, which is concomitant with decreased Notch2-Dll1 signaling, prevents formation of a mixed population of chondrocytes and tenocytes, and instead results in ectopic endochondral bone at tendon-bone attachment units. Our work shows that FGF signaling directs zonal patterning at the boundary between tendon and bone by regulating cell fate decisions through a mechanism that employs Notch signaling.
KEY WORDS: FGF, Notch, Craniofacial development, Enthesis, Perichondrium, Tendon, Mouse
Summary: FGF signaling is essential in establishing the graded transitional tissue of the tendon-bone attachment unit by regulating cell fate decisions and the development of Scx+/Sox9+ cells.
INTRODUCTION
Body movement requires tendons to deliver high tensile forces from skeletal muscle to bone. As tendon and bone have vastly different physical properties, a transitional connective tissue called the enthesis allows for smooth transfer of mechanical load across their interface. The enthesis dissipates stress concentrations and strengthens bonding at the soft-hard tissue interface through its zonal architecture of tendon, fibrocartilage, mineralized fibrocartilage and bone (Benjamin et al., 2006). Although this gradient of cell types, collagen fibers and mineral is adapted to withstand mechanical stress induced by movement, the structural integrity of the enthesis is vulnerable to rheumatic disease and overuse injuries (Lu and Thomopoulos, 2013; Thomopoulos et al., 2002). Following damage, entheses repair through a persistent fibrovascular scar that lacks the structural and functional properties of the native tendon-bone attachment (Lu and Thomopoulos, 2013). To advance clinical strategies for enthesis repair and regeneration, it is important to gain a more complete understanding of the mechanisms that establish tissue complexity at the tendon-bone interface during development.
The graded structure of the enthesis has its origins in the embryonic tendon-bone attachment unit, which is composed of the tendon's tip and the bone eminence into which it inserts. Soon after formation of the cartilage anlage that prefigures the bone, an adjacent pool of progenitors co-expressing scleraxis (Scx) and Sox9 (Scx+/Sox9+) are specified through an independent and little-understood mechanism that requires TGFβ signaling (Blitz et al., 2013). As skeletal development proceeds, Scx+/Sox9+ progenitors near the tendon differentiate into Scx+ tenocytes, whereas those near the bone form Sox9+ chondrocytes of the bone eminence (Akiyama et al., 2005; Soeda et al., 2010; Sugimoto et al., 2013). Formation of the eminence is induced by Bmp4, which emanates from Scx+ cells and promotes differentiation of Scx+/Sox9+ progenitors into chondrocytes (Blitz et al., 2009). Scx+/Sox9+-derived chondrocytes of the eminence subsequently undergo endochondral-like ossification to form the bony end of the mature enthesis. During late embryonic stages, Gli1+ cells emerge within the developing tendon-bone attachment and contribute to the mature enthesis in the adult (Dyment et al., 2015; Felsenthal et al., 2018; Schwartz et al., 2015). In stationary entheses at the ends of bones, Gli1+ cells are derived from the embryonic Sox9-lineage and give rise to fibrocartilage and mineralized fibrocartilage (Felsenthal et al., 2018). These mechanisms that regulate the development of tendon-bone attachment units have been described in the context of the appendicular skeleton; however, the extent to which these molecular mechanisms are conserved in the craniofacial complex has not yet been determined.
In the craniofacial complex, jaw movement is driven by the transmission of muscle force across tendons focally inserted on the mandible's three proximal processes: the coronoid, the condyle and the angular process. In mice, the condyle and angular process form as accessory condensations of secondary cartilage that cap the intramembranous mandible to facilitate growth at the temporomandibular joint (TMJ) (Anthwal and Tucker, 2012; Hall, 2015). The secondary cartilages of the condylar and angular processes have shared characteristics with cartilages that form long bone eminences in the limb: they are specified by TGFβ signaling, maintained by the mechanical stimuli from the surrounding musculature, express collagen (Col) -I and -II in their perichondrial layers and undergo endochondral-like ossification (Anthwal et al., 2008; Hinton and Carlson, 2005; Rot-Nikcevic et al., 2007; Shibukawa et al., 2007). In the avian jaw, specification of secondary cartilage requires not only TGFβ signaling, but also FGF signaling through Fgfr2 (Solem et al., 2011; Woronowicz et al., 2018). Although Fgfr2 is highly localized to articular and perichondrial layers of the mouse condylar cartilage, its role in the mammalian jaw has not yet been determined (Fuentes et al., 2002). Nevertheless, Fgfr2 signaling plays a key role in mammalian skeletal development. Fgfr2 is among the earliest genes expressed in condensing skeletogenic mesenchyme and, upon initiation of skeletogenesis, its expression becomes restricted to the progenitor-laden tissue of perichondrium/periosteum (Ornitz and Marie, 2015). FGFR2 mutations disrupt perichondrial/periosteal development by altering the balance of proliferation and differentiation in skeletal progenitors (Eswarakumar et al., 2004, 2002; Merrill et al., 2012; Yu et al., 2003). As the perichondrium/periosteum lies at the tendon-bone interface, Fgfr2 signaling likely regulates tendon-bone attachment unit development.
Here, we show that secondary cartilages of the mandibular condyle and angular process, as with long bone eminences in the limb, are derived in part from the Scx-lineage and require Scx for their development. We also demonstrate that the contribution of Scx+ cells to the secondary cartilages is crucial for development of tendon-bone attachment units and is regulated by Fgfr2. Loss of Fgfr2 in neural crest cell (NCC)-derived skeletal progenitors of the mandible alters development of Scx+/Sox9+ cells and biases their differentiation into chondrocytes through a mechanism that disrupts Notch-Dll1 signaling. Together, these results identify a crucial role for FGF signaling in establishing the graded transitional tissue of the tendon-bone attachment unit.
RESULTS
Scx+ cells contribute to tendon-bone attachment units in the mandible
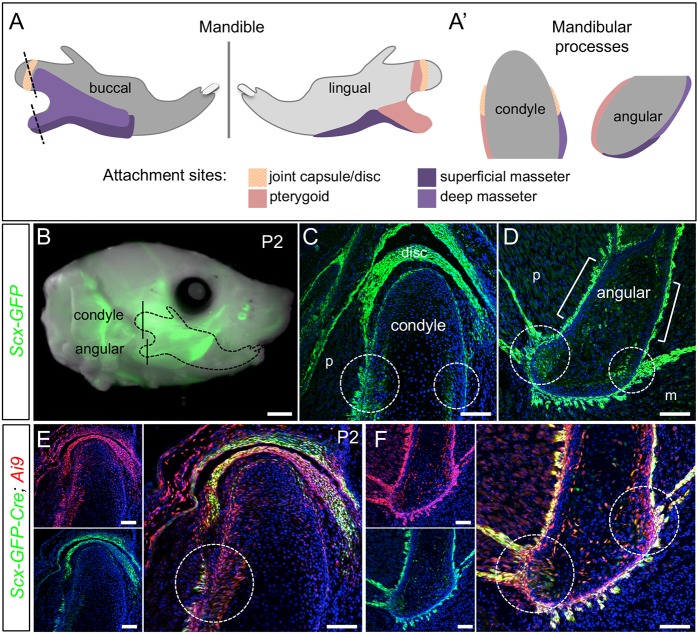
The condyle and angular process are key tendon attachment sites for masticatory muscles, including the pterygoid and masseter (Fig. 1A-A′) (Baverstock et al., 2013). Although the tendon-bone attachments that anchor these muscles to the mandible are histologically described in humans, this description is lacking in mice (Hems and Tillmann, 2000). As the Scx-GFP tendon reporter marks periodontal ligament-to-bone entheses in mice (Lee et al., 2015; Pryce et al., 2007), we used this line to characterize tendon-bone attachments in the mandible. Whole-mount and histological sections of the condyle and angular process at postnatal day (P)2 showed Scx+ cells at the insertions for force-transmitting tendons and muscle-anchoring tendons (n=3) (Fig. 1B-D). In insertions for force-transmitting tendons, Scx+ cells marked the long tendon fibers, as well as the perichondrium and underlying cartilaginous zone (Fig. 1C-D, circles). A limited number of Scx+ cells were also found within the hypertrophic and mineralized zones of the angular process. In insertions for muscle-anchoring tendons, Scx+ cells marked short tendon fibers and the outer perichondrial layer of the condyle and angular process (Fig. 1C,D, brackets). Lineage tracing using Scx-Cre; Ai9 confirmed that Scx+ cells contribute to not only the tendon, but also the perichondrium and cartilaginous zone of the condyle and angular process by P2 (n=2) (Fig. 1E,F, circles).
Fig. 1.
Scx-GFP and Scx-Cre mark tendon insertions in the mandible. (A,A′) Diagrams indicate attachment sites for the muscles of mastication and the temporomandibular joint capsule on the buccal and lingual sides of the mandible. Dotted lines indicate coronal plane of section for the condyle and angular process in A′. (B) Whole-mount of Scx-GFP at P2 identifies the craniofacial tendons (n=3). (C,D) Coronal sections through the condyle (C) and angular process (D) of Scx-GFP mice at P2, as indicated in B, show the insertion sites for force-transmitting tendons (circles) and muscle-anchoring tendons (brackets) (n=3). (E,F) Coronal sections through the condyle (E) and angular process (F) of Scx-Cre; Ai9 mice at P2 show the contribution of Scx+ cells to cartilage and bone at the tendon insertion (circles) (n=2). m, masseter; p, pterygoid. Scale bars: 1 mm (whole mount); 100 μm (sections).
Scx regulates development of tendon-bone attachment units in the mandible
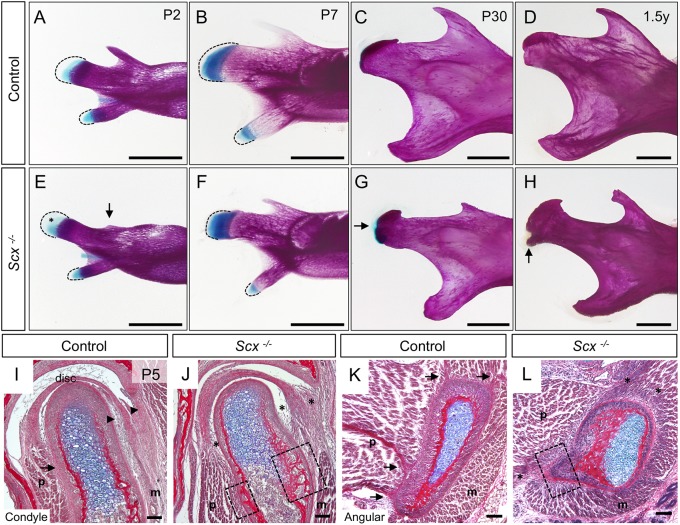
To determine the functional role of Scx in the development of mandible tendon-bone attachment units, we examined the condyle and angular process in Scx knockout mice. Whole-mount skeletal preparations of Scx−/− mandibles at P2 identified ectopic Alcian Blue stain within the secondary cartilage of the condyle compared with controls (n=3) (Fig. 2A,E, asterisk). Scx−/− mandibles also exhibited delayed development of the coronoid process, a site of direct tendon insertion for the temporalis muscle, which forms independent from secondary cartilage (Fig. 2E, arrow) (Anthwal et al., 2008). At P7, during endochondral-like ossification of the secondary cartilages, Scx−/− mandibles showed a progressive increase in ectopic Alcian Blue stain within the prechondrogenic mesenchyme of the condyle and angular process compared with controls (n=2) (Fig. 2B,F). At P30, following secondary cartilage ossification, the condyle and angular process of Scx−/− mandibles were dysmorphic and the articular surface of the condyle was topped with ectopic cartilage (n=3) (Fig. 2C,G, arrow). Morphological changes in the condyle and angular process in 1.5 year old Scx−/− mice were associated with osteophyte formation, a hallmark of joint damage (n=2) (Fig. 2D,H, arrow). Histological analyses at P5 showed that tendon insertions linking the masseter and pterygoid muscles to the perichondrial surface of the condyle and angular process were incomplete and/or missing in Scx−/− mice (n=3) (Fig. 2I,L, arrows versus asterisks). In addition, insertion for the tendinous lamina connecting the articular disc to the condyle and zygomatic arch were deficient (Fig. 2I,J, arrowheads versus asterisks). In regions where tendon insertion was disrupted, ectopic bone formed on the outer layer of the mandibular processes (Fig. 2J,L, boxes). These data demonstrate that Scx is necessary for proper development of the mandibular processes and their tendon insertions.
Fig. 2.
Scx regulates development of the mandibular processes and their tendon insertions. (A-H) Whole-mount skeletal preparations of mandibles from control (A-D) and Scx−/− littermates (E-H). Dashed lines mark the mesenchyme. (A,E) At P2, Scx−/− mandibles have an underdeveloped coronoid process (arrow) and ectopic Alcian Blue stain (asterisk) in the condylar mesenchyme (n=3). (B,F) At P7, the Scx−/− mandibles show precocious expansion of Alcian Blue stain in the condyle and angular process (n=2). (C,G) At P30, the Scx−/− mandibles exhibit ectopic Alcian Blue stain on the articular surface of the condyle (arrow), as well as a bulbous angular process (n=3). (D,H) By 1.5 years, the Scx−/− condyles develop a bony protrusion near the insertion site of the joint capsule (arrow) (n=2). (I-L) Coronal sections of the condyle (I,J) and angular process (K,L) stained with HBQ at P5. Tendon insertions in the controls (arrows) are disrupted in Scx−/− mice (asterisks). Articular disc attachments are disrupted in Scx−/− mice (arrowheads versus asterisks) (n=3 littermate pairs). Ectopic bone develops on the condyle and angular process at the sites of tendon insertions (boxes). m, masseter; p, pterygoid. Scale bars: 1 mm (skeletal preparations); 100 μm (sections).
Fgfr2 regulates development of tendon-bone attachment units in the mandible
FGF signaling, likely through Fgfr2, regulates specification of the secondary cartilages in the avian jaw (Solem et al., 2011; Woronowicz et al., 2018). To identify a role for Fgfr2 in development of the mammalian mandibular processes and their tendon insertions, we first examined receptor expression at embryonic day (E) 16.5, soon after the secondary cartilages form. Fgfr2 was expressed throughout the condyle and angular process, as well as in the surrounding perichondrial region (Fig. S1A). This pattern of Fgfr2, consistent with previous reports, was maintained at E18.5 and P2 (Fig. S1B,C) (Purcell et al., 2012).
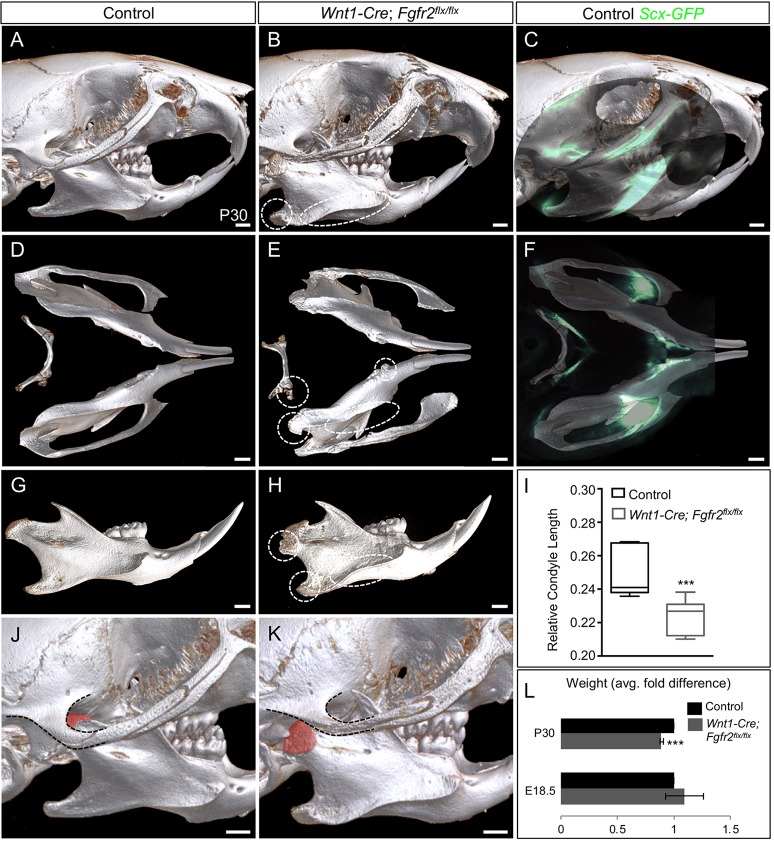
Genetic lineage tracing using the Wnt1-Cre driver previously showed that NCC-derived mesenchyme gives rise to mandible secondary cartilage, tendons and tendon-bone attachment units (Matsuoka et al., 2005; Yu et al., 2003). We confirmed these findings in the angular process of Wnt1-Cre; Ai9; Scx-GFP mice at P2 (Fig. S1D). To determine the necessity of Fgfr2 in the development of the mandibular processes and their tendon insertions, we examined the skulls of Wnt1-Cre; Fgfr2flx/flx mice. Analysis by micro-computed tomography (μCT) at P30 identified ectopic bone on the mandibular ramus, condyle and angular process as well as on the zygomatic arch and hyoid in mutant mice (n=3 littermate pairs) (Fig. 3A,B,D,E,G,H, circles and ellipses). Whole-mount overlay of Scx-GFP on the μCT renderings showed that regions of ectopic bone in the mutant corresponded to tendon insertions in the control (n=2) (Fig. 3C,F). Morphometric analyses at P30 showed that the mandibular height (P<0.001) and lengths (P<0.001 and P<0.001), and condylar length (P<0.001) were reduced in Wnt1-Cre; Fgfr2flx/flx mice compared with controls (n=6 same-sex littermate pairs) (Fig. S2A,D,G; Fig. 3I). Wnt1-Cre; Fgfr2flx/flx mice exhibited abnormalities in the TMJ including ectopic bone formation on the condyle at the site of tendon insertion for the pterygoid, joint dislocations and osteophyte formation (n=3 littermate pairs) (Fig. 3J-K; Fig. S2B,C,E,F, arrows). The jaw defects in Wnt1-Cre; Fgfr2flx/flx mice at P30 coincide with diminished body weight compared with controls (n=6 same-sex littermate pairs) (P<0.001), suggesting compromised mastication (Fig. 3L). The coronoid process appeared to be relatively normal in Wnt1-Cre; Fgfr2flx/flx mandibles (Fig. 3G,H). Taken together, these data show that Fgfr2 regulates bone formation at the tendon-bone interface in multiple regions of the face and jaw, including those of the condyle and angular process.
Fig. 3.
Loss of Fgfr2 induces ectopic bone at tendon insertions. (A-H) μCT analyses at P30 show that Wnt1-Cre; Fgfr2flx/flx skulls develop ectopic bone (ellipses) compared with controls (n=3). Whole-mount analysis of Scx-GFP overlaid on control μCT renderings (C,F) correlates ectopic bone with tendon insertion sites (n=2). (I) Morphometric analysis of condyle length in Wnt1-Cre; Fgfr2flx/flx mice compared with controls (n=6 same-sex littermate pairs). (J,K) The condyle's articular surface (pseudo-colored) is dislocated from the temporal bone (dashed outline) in the mutant TMJ (K) compared with the control (J). (L) The average fold difference in weight calculated in control and Wnt1-Cre; Fgfr2flx/flx at E18.5 and P30 (n=6 same-sex littermate pairs). ***P<0.001 (unpaired two-tailed t-tests). Error bars indicate s.e.m. Scale bars: 1 mm.
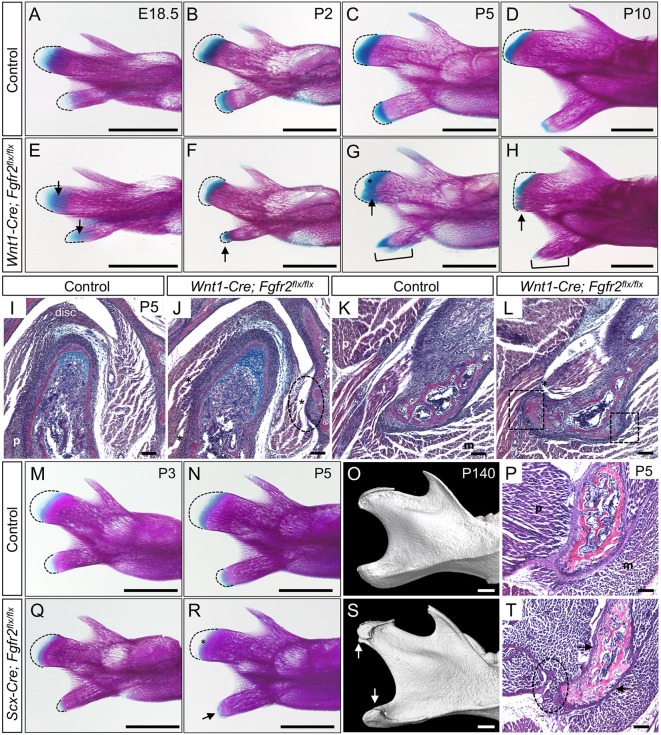
To determine the timing of ectopic bone formation at tendon-bone attachment units in Wnt1-Cre; Fgfr2flx/flx mice, we analyzed a series of staged mandibles by whole-mount skeletal preparation. At E18.5, condylar and angular secondary cartilages in Wnt1-Cre; Fgfr2flx/flx mandibles had a slight increase in Alcian Blue stain compared with controls (n=3 littermate pairs) (Fig. 4A,E, arrows). At P2, the Wnt1-Cre; Fgfr2flx/flx angular process was smaller, and the region of Alcian Blue stain was abnormally shaped compared with controls (n=3 littermate pairs) (Fig. 4B,F, arrow). By P5, the angular process mesenchyme was prematurely lost, and ectopic bone spicules appeared at tendon-insertion sites in the mutant (n=3 littermate pairs) (Fig. 4C,G, bracket). In the mutant condyle at P5, ectopic Alcian Blue stain and premature advancement of the mineralized bone front into the mesenchyme was observed (Fig. 4C,G, asterisk, arrow). At P10, the Wnt1-Cre; Fgfr2flx/flx angular process had prematurely mineralized and was encapsulated in ectopic bone (n=3 littermate pairs) (Fig. 4D,H, bracket). The mutant condyle showed premature loss of the growth plate, as well as ectopic bone near the region of tendon insertion (Fig. 4D,H, arrow).
Fig. 4.
Fgfr2 regulates development of the mandibular processes and their tendon insertions. (A-H) Whole-mount skeletal preparations of mandibles from control (A-D) and Wnt1-Cre; Fgfr2flx/flx littermates (E-H). Dashed lines mark the prechondrogenic mesenchyme. (A,E) At E18.5, there is increased Alcian Blue in the mesenchyme of the Fgfr2 mutant condyle and angular process (arrows). (B,F) At P2, the mutant angular process is abnormally shaped and its mesenchyme is ectopically stained with Alcian Blue (arrow). (C,G) In the mutant at P5, ectopic bone spicules form at sites of tendon attachment (bracket) in angular process and ectopic Alcian Blue marks the growing front of the condyle (arrow, asterisk). (D,H) At P10, the mutant angular process is prematurely mineralized (bracket), and ectopic bone is seen at tendon attachment sites in the condyle (arrow). (I-L) Histological sections of the condyle (I,J) and angular process (K,L) stained with HBQ at P5. (I,J) In the mutant condyle, tendon insertion sites for the pterygoid, masseter and disc are incomplete (ellipse) compared with control. (K,L) In the mutant angular process, insertion sites for tendons (t) are incomplete (boxes) and contain ectopic bone (asterisk). (M-T) Whole-mount skeletal preparations at P3 (M,Q) show that the angular process of Scx-Cre; Fgfr2flx/flx mice is dysmorphic with premature loss of mesenchyme (outlined). At P5 (N,R), the cartilage of Scx-Cre; Fgfr2flx/flx mice is prematurely lost in the angular process (arrow) and condyle (asterisk). (O,S) μCT analyses at P140 identify ectopic bone on the condyle and angular process of Scx-Cre; Fgfr2flx/flx mice (arrows) (n=3 littermate pairs). (P,T) Histological sections of the angular process stained with HBQ at P5 show that tendon insertions are incomplete (ellipse) and the perichondrium is thin (arrows) in the mutant. n=3 littermate pairs for each. m, masseter; p, pterygoid. Scale bars: 1 mm (skeletal preparations); 100 µm (sections).
Histological analyses of the condyle and angular process at P5 in Wnt1-Cre; Fgfr2flx/flx mice showed abnormalities in tendon insertion (n=3 littermate pairs). The tendon attachment between the articular disc and the temporal bone in Wnt1-Cre; Fgfr2flx/flx mice was largely missing and instead the region was occupied by ectopic bone (Fig. 4,J, ellipse, asterisk). Tendon attachments linking the superior and inferior bellies of the pterygoid to the condyle and the disc, respectively, were disrupted in mutants (Fig. 4I,J, asterisks). Tendon attachments linking the pterygoid and masseter muscles to the mutant angular process were incomplete and exhibited ectopic bone (Fig. 4K,L, asterisk, boxes). Together these findings show that sites of tendon-bone insertion are disrupted and instead occupied by ectopic bone in the Fgfr2 mutant mandible.
To identify a requirement for Fgfr2 in Scx+ cells in the mandibular processes and their tendon insertions, we conditionally knocked out Fgfr2 using the Scx-Cre driver. Whole-mount skeletal preparations at P3 showed the Scx-Cre; Fgfr2flx/flx angular process was truncated (n=3 littermate pairs) (Fig. 4M,Q). By P5, the Scx-Cre; Fgfr2flx/flx angular process was prematurely ossified, and Alcian Blue stain in the condyle was reduced (n=3) (Fig. 4N,R, arrow, asterisk). μCT analyses at P140 identified ectopic bone on the condyle and angular process of Scx-Cre; Fgfr2flx/flx mice (n=3) (Fig. 4O,S, arrows). Morphometric analyses at P30 showed that, although the length of the condyle in Scx-Cre; Fgfr2flx/flx mice was shorter compared with control (P<0.05), the difference in condylar length relative to the overall length of the mandible was not statistically significant (P=0.12) (n=3 same-sex littermate pairs) (Fig. S2H,I). Histological sections at P5 revealed that the perichondrium was reduced and tendon insertions were incomplete in the mutant angular process (Fig. 4P,T, arrows, ellipse). Together this data demonstrates that Fgfr2 regulates Scx-lineage cells within the condyle and angular process.
Fgfr2 regulates bone formation at the tendon-bone interface
To further examine the mechanism leading to ectopic bone formation within the tendon-bone attachment units of Wnt1-Cre; Fgfr2flx/flx mice, we focused our analyses on the angular process, as its primary function is for muscle attachment, unlike the joint-articulating condyle. Histological staining of un-decalcified sections at P5 with Toluidine Blue to detect cartilage proteoglycans and von Kossa to detect calcium mineralization were used to identify the type of ossification leading to ectopic bone formation in the mutant angular process. Toluidine Blue and von Kossa dually labeled the ectopic bone in the perichondrial layers of the Wnt1-Cre; Fgfr2flx/flx angular process (n=3 littermate pairs) (Fig. S3A-D, arrows), indicating that the ectopic bone formed through endochondral ossification.
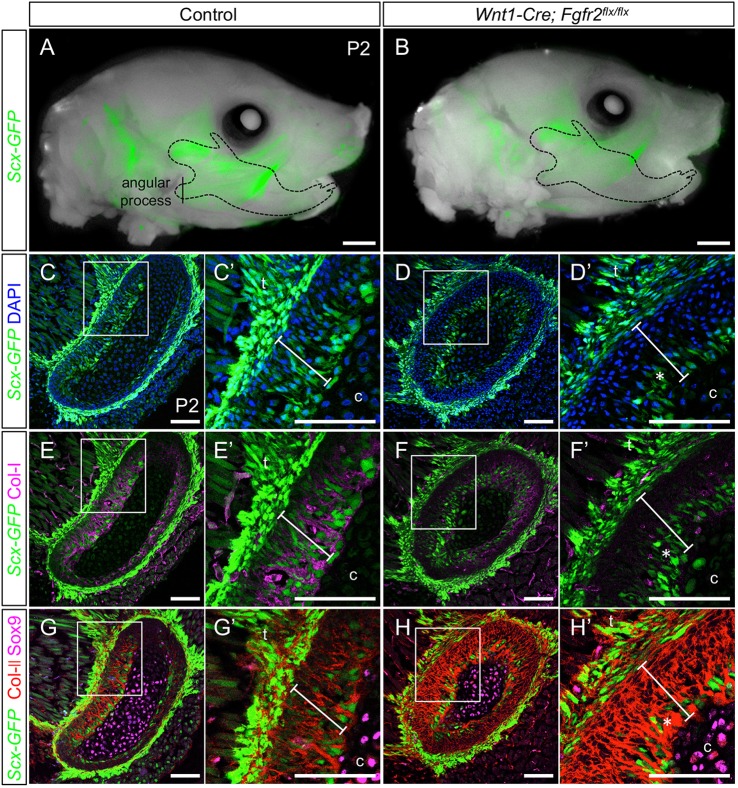
FGF signaling modulates Scx expression to regulate tenocyte differentiation in the developing limb (Brent et al., 2003; Brent and Tabin, 2004; Havis et al., 2016). Therefore, we examined the relationship between ectopic bone in the Fgfr2 mutant and Scx+ cells in the angular process. The Scx-GFP reporter, along with Col-I and Col-II immunofluorescence at P2, confirmed that the outer perichondrial layer of the control angular process co-expressed Col-I and Col-II and was populated by a mixture of Scx+ and Scx− cells (n=3) (Fig. 5A,C,C′,E,E′,G,G′, bars), an expected outcome as Scx promotes Col1a1 expression (Murchison et al., 2007). The angular cartilage core, on the other hand, expressed Sox9, low levels of Col-II and was negative for Col-I and Scx-GFP (n=3) (Fig. 5E,E′,G,G′). This is consistent with studies showing that the condyle is composed of an inner core of cartilage surrounded by fibrocartilage (Chen et al., 2012; He et al., 2017; Hinton and Carlson, 2005). In the Wnt1-Cre; Fgfr2flx/flx angular process, Scx-GFP was reduced in the perichondrial layer and Scx+ cells were restricted to a small layer around the core cartilage (n=3) (Fig. 5B,D,D′, asterisk). Correspondingly, Col-I was diminished and Col-II was expanded in the perichondrium of the mutant angular process (n=3) (Fig. 5F,F′,H,H′, bars). Expression of Sox9 and Col-II in the cartilage core of the mutant angular process was similar to the control (Fig. 5H,H′). Together these findings show that Fgfr2 promotes the contribution of Scx+ cells to the perichondrial layers of the angular process.
Fig. 5.
Fgfr2 promotes formation of Col-I+/Col-II+ region at tendon-bone attachments. (A,B) Whole-mount analyses (fluorescent and brightfield images overlaid) at P2 show reduced Scx-GFP in the mandible (dashed outline) of Wnt1-Cre; Fgfr2flx/flx mice (B) compared with control (A). (C,C′) Coronal sections through the control angular process at P2 (vertical line in A) shows Scx-GFP in the control tendon, as well as in cells at the tendon-bone insertion, which lay between the tendon and the cartilage core of the angular process (bar). (D,D′) In the P2 Wnt1-Cre; Fgfr2flx/flx angular process, Scx-GFP is reduced in the outer layer of the angular process (asterisk). (E-F′) Immunofluorescence reveals that decreased Col-I coincides with decreased Scx-GFP in the mutant angular process (asterisk). (G-H′) Immunofluorescence for Col-II and Sox9 shows that decreased Scx-GFP in the mutant angular process coincides with increased Col-II (asterisk). n=3 littermate pairs for each. C′,D′,E′,F′,G′,H′ are digital magnifications of the boxed regions in C,D,E,F,G,H, respectively. c, cartilage core; t, tendon. Scale bars: 1 mm (whole mount); 100 µm (sections).
Fgfr2 regulates development of Scx+/Sox9+ progenitors
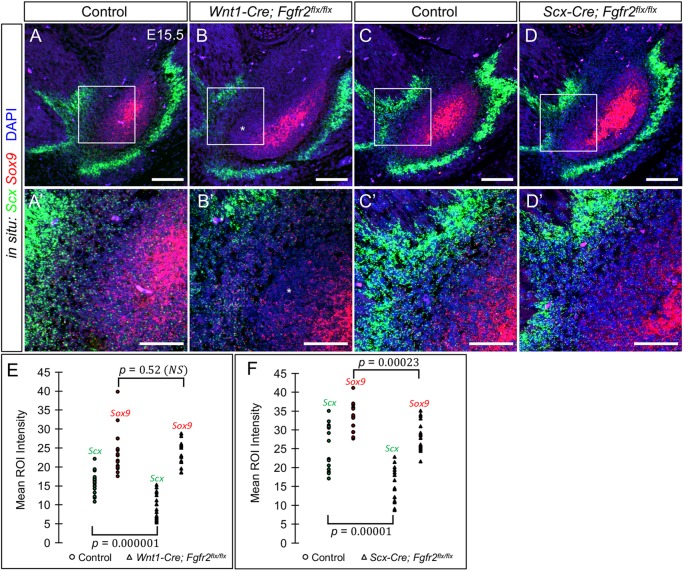
In the limb, Scx+/Sox9+ cells form tendon-bone attachment units; however, the extent to which these cells contribute to tendon-bone attachments in the craniofacial complex has not been determined. Double fluorescent RNA in situ hybridization on the angular process at E15.5, when as the secondary cartilage emerges, identified cells co-expressing Scx and Sox9 in the perichondrial region between Sox9+ cells of the cartilage core and Scx+ cells of the tendon (n=3) (Fig. 6A,A′). Scx+/Sox9+ cells were also identified in the perichondrial region of the Wnt1-Cre; Fgfr2flx/flx angular process (Fig. 6B,B′). However, Scx levels were significantly reduced (n=3) (P=0.000001), whereas Sox9 levels remained unchanged (P=0.52) (Fig. 6E). Ectopic cells expressing low levels of Sox9 were located between Scx+/Sox9+ cells and the perichondrium (Fig. 6B,B′, asterisks). This suggests that Fgfr2 is crucial for Scx expression in the Scx+/Sox9+ precursors of tendon-bone attachment units. We next examined the role of Fgfr2 following Scx+/Sox9+ specification. In the angular process of Scx-Cre; Fgfr2flx/flx embryos at E15.5, both Scx and Sox9 levels were reduced in Scx+/Sox9+ cells compared with controls (n=3) (P=0.00001 and P=0.00023) (Fig. 6C,C′,D,D′,F). This suggests that Fgfr2 is essential for maintaining the Scx+/Sox9+ cells. Altogether, these results confirm that Fgfr2 regulates early development of Scx+/Sox9+ cells at the tendon-bone interface in the mandible.
Fig. 6.
Fgfr2 regulates Scx and Sox9 expression within the tendon-bone interface. (A-D′) Double fluorescent in situ hybridization for Scx (green) and Sox9 (red) in the angular process at E15.5 (n=3 littermate pairs). (A,A′,C,C′) The control angular process shows high expression of Scx within the tendon and Sox9 within the angular cartilage. Cells in the perichondrial region express both Scx and Sox9 (A′). (B-B′) The Wnt1-Cre; Fgfr2flx/flx angular process shows cells co-expressing Scx and Sox9 in the perichondrial region, in addition to an ectopic population of Sox9-expressing cells (asterisk). (C-C′,D-D′) The Scx-Cre; Fgfr2flx/flx angular process exhibit a reduction of Scx and Sox9 expression in the perichondrial region compared with controls. A′,B′,C′,D′ show 63× magnification of the boxed regions in A,B,C,D, respectively. (E) Quantification of the fluorescent intensity of Scx and Sox9 in the double-positive region shows that Wnt1-Cre; Fgfr2flx/flx angular process have significantly lower levels of Scx expression (P=0.000001), whereas Sox9 levels remain unchanged (P=0.52). (F) Quantification of the fluorescent intensity of Scx and Sox9 in the double-positive region shows that Scx-Cre; Fgfr2flx/flx angular process have reduced Scx (P=0.00001) and Sox9 (P=0.00023) expression (two-tailed t-test). Scale bars: 100 µm in A-D; 50 µm in A′-D′.
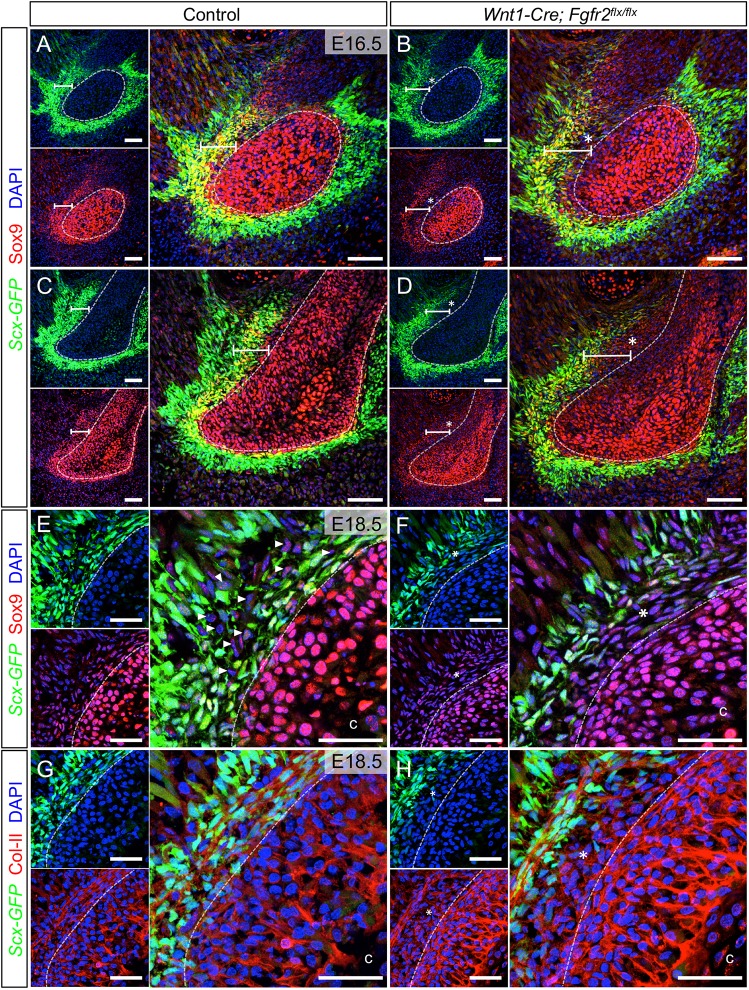
To further examine the development of Scx+/Sox9+ cells in the angular process, we used Scx-GFP and Sox9 immunofluorescence at E16.5. In the control angular process, Scx+/Sox9+ progenitors occupied the tendon-bone interface between Scx+ cells of the tendon and Sox9+ cells of the prechondrogenic core (n=3) (Fig. 7A, bar). The angular perichondrium, demarcated by a dotted line, was distinguishable histologically (Fig. S4A,B). In the Wnt1-Cre; Fgfr2flx/flx angular process, Scx+/Sox9+ progenitors lay between the perichondrium and tendon; however, an interposing cell population expressing low levels of both Scx and Sox9 displaced them from the surface of the perichondrium (n=3) (Fig. 7B, asterisk). In more-developed regions located rostrally within the control angular process, Scx+/Sox9+ cells were interspersed with Scx+ and Sox9+ single-positive cells (n=3) (Fig. 7C, bar). This pattern is consistent with previous studies suggesting bipotent Scx+/Sox9+ progenitors differentiate into cells that are single-positive for Scx and Sox9 in developing tendon-bone attachment units (Sugimoto et al., 2013). In the rostral angular process of the mutant, fewer Scx+/Sox9+ cells were observed, concomitant with an increase in Sox9+ cells (n=3) (Fig. 7D, bar, asterisk). Notably, there was also less mixing between cells that were single-positive for Scx or Sox9 around the angular perichondrium. In the coronoid process, we did not find evidence of Scx+/Sox9+ progenitors (n=3) (Fig. S4C).
Fig. 7.
Loss of Fgfr2 alters development of Scx+/Sox9+ progenitors and induces their biased differentiation into chondrocytes. (A) In the control angular process at E16.5, Scx-GFP and Sox9 immunofluorescence identify Scx+/Sox9+ cells (yellow, bar) between Scx+ cells of the tendon (green) and Sox9+ cells of the angular cartilage core (red, dashed outline). (B) In the Wnt1-Cre; Fgfr2flx/flx angular process, Scx+/Sox9+ cells are present, but loosely dispersed (bar) and separated from the angular cartilage core by a layer of Scx− cells expressing low levels of Sox9 (asterisk). (C) In rostral regions of the angular process, which is more developed, cells individually expressing Scx-GFP or Sox9 are found interspersed with Scx+/Sox9+ cells (bar) at the tendon-bone interface. (D) In the mutant angular process, Sox9+ cells are more numerous (asterisk) and fewer Scx+/Sox9+ cells are seen (bar). (E) In the control angular process at E18.5, Sox9 immunofluorescence and Scx-GFP identify Sox9+ cells (arrowheads) interspersed between Scx+ and Scx+/Sox9+ cells. (F) In the Wnt1-Cre; Fgfr2flx/flx angular process, the majority of the cells are Sox9+ (asterisk). (G) Col-II immunofluorescence in the control angular process marked the cartilage core and the Scx-GFP+ outer layer. (H) In the Fgfr2 mutant angular process, Col-II is in the cartilage core, Scx-GFP+ regions of the tendon-bone insertion and the ectopic domain found to express low levels of Sox9 (asterisk). Smaller panels show individual fluorescent channels with DAPI. n=3 littermate pairs for each. Dashed line demarcates angular core cartilage. c, cartilage core. Scale bars: 100 µm in A-D; 50 µm in E-H.
In the control angular process at E18.5, after the secondary cartilage undergoes appositional growth and maturation, a diminished population of Scx+/Sox9+ progenitors remained in the perichondrium. On the tendon side of Scx+/Sox9+ progenitors lay a mixture of Scx+ and Sox9+ single-positive cells of the developing tendon-bone attachment (n=3) (Fig. 7E, arrowheads). In the Wnt1-Cre; Fgfr2flx/flx angular process at E18.5, a majority of the cells in tendon-bone attachment were Sox9+ (n=3) (Fig. 7F, asterisk). Col-II expression showed that Sox9+ cells in the mutant angular process had prematurely acquired a chondrogenic phenotype (n=3) (Fig. 7G,H, asterisk). These findings suggest Fgfr2 promotes formation of the mixed region of Scx+ and Sox9+ single-positive cells in the developing tendon-bone attachment and that loss of Fgfr2 induces biased differentiation of Scx+/Sox9+ cells into Sox9+ chondrocytes.
Fgfr2 and Fgf2 expression domains are complementary at the tendon-bone interface
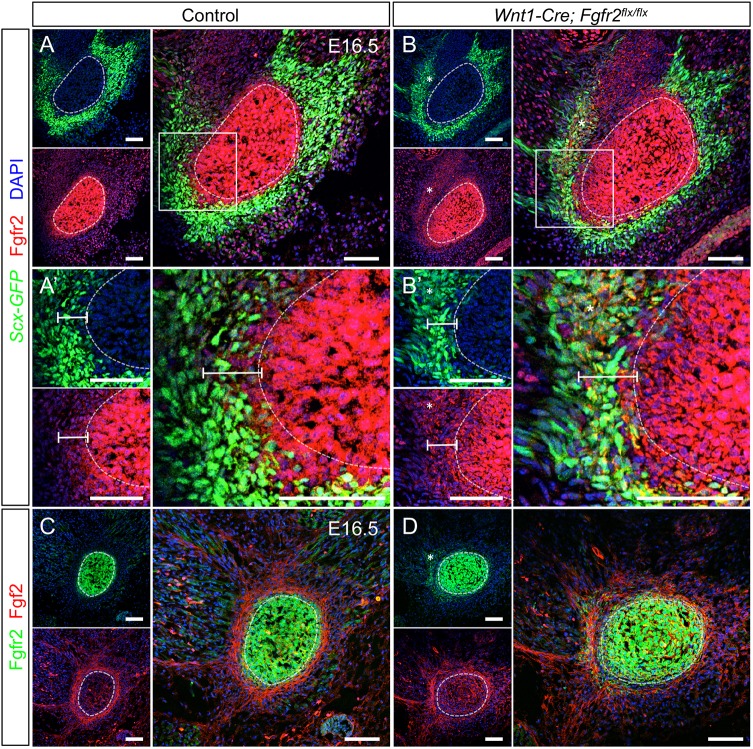
In order to identify regions of FGF signaling within the developing tendon-bone interface, we examined localization of Fgfr2 and its ligand Fgf2 in the angular process at E16.5. In control, Fgfr2 was expressed by cells in and around the angular cartilage and in the muscular connective tissue (n=3) (Fig. 8A). Fgfr2+ cells in the perichondrium of the angular process overlapped with the Scx+ region (Fig. 8A′, bar). In the tendon, where cells strongly expressed Scx-GFP, Fgfr2 was low (Fig. 8A,A′). In control embryos, Fgf2 was highly expressed within the cellular extensions of tenocytes (n=3) (Fig. 8C). Fgf2 in the angular cartilage was relatively low. These results are consistent with previous studies showing that Fgf2 is concentrated in the cell layers surrounding the condyle (Ogawa et al., 2003).
Fig. 8.
Fgfr2 and Fgf2 expression patterns are complementary at the tendon-bone interface. (A) In the control at E16.5, Fgfr2 is high in and around the angular process and within the muscular connective tissue. (A′) Digital zoom of boxed area in A showing Fgfr2 in the Scx-GFP+ region populated by Scx+/Sox9+ progenitors (see Fig. 9A,C). (B) In the Wnt1-Cre; Fgfr2flx/flx angular process, Fgfr2 knockout cells are more widely dispersed than in the control (asterisk). (B′) Digital zoom of boxed area in B showing that Fgfr2 knockout cells are ectopically located in the Scx-GFP+ region of the tendon (bar, asterisk). (C) In the control, Fgf2 is high in the tendon and a ring of cells surrounding the angular process. (D) In the Fgfr2 mutant, the ring of Fgf2-expressing cells is irregular and displaced from the angular cartilage core. Fgf2 is also upregulated in the angular cartilage core. Smaller panels show individual fluorescent channels with DAPI. n=3 littermate pairs for each. Scale bars: 100 μm.
As Cre recombination of the Fgfr2flx allele generates a truncated non-functional protein product that is still recognized by the Fgfr2 antibody (Fig. S5), we used immunofluorescence to identify the location of Fgfr2 knockout cells within the mutant. In Wnt1-Cre; Fgfr2flx/flx embryos, the Fgfr2 knockout cells were ectopically located outside the angular process in the tendon and muscular connective tissue (n=3) (Fig. 8B,B′,D, bar, asterisks). Fgf2 localization was disorganized and displaced from the angular process by Fgfr2 knockout cells in the Wnt1-Cre; Fgfr2flx/flx angular process (n=3) (Fig. 8D). Fgf2 was also upregulated through the angular cartilage core (Fig. 8D). Spatiotemporal localization of Fgfr2 and its ligand Fgf2 in the angular process shows that the tendon-bone interface, which houses Scx+/Sox9+ progenitors, has potential for active FGF signaling during key stages of tendon-bone attachment unit formation.
Fgfr2 regulates Notch signaling at the tendon-bone interface
Interplay between FGF and Notch signaling maintains progenitors and determines cell fate in multiple tissue types (Akai et al., 2005; Millimaki et al., 2007; Tummers and Thesleff, 2003; Wahl et al., 2007). These findings, along with the well-documented role of Notch signaling in binary cell fate choice, prompted us to investigate the possibility that Fgfr2 regulates Scx+/Sox9+ cell fate choice via the Notch pathway. In the angular process of controls at E16.5, Notch2 was expressed in the core cartilage, perichondrium, tendon-bone interface and muscular connective tissue (n=3) (Fig. 9A). In the Wnt1-Cre; Fgfr2flx/flx mice, the Notch2 levels throughout the angular process and muscle connective tissue were reduced (n=3) (Fig. 9B). Quantitative RT-PCR of mRNA isolated from angular processes at E16.5 confirmed that transcripts for Notch2 (P<0.05), as well as for its targets Hes1 (P<0.05) and Hes7 (P<0.01), were significantly lower in the mutant compared with control (n=3 littermate pairs) (Fig. S6A). Relative transcript levels of the Notch target Hes5, on the other hand, were not statistically different. These data demonstrate that loss of Fgfr2 correlates with an overall decrease in Notch2 signaling in the angular process.
Fig. 9.
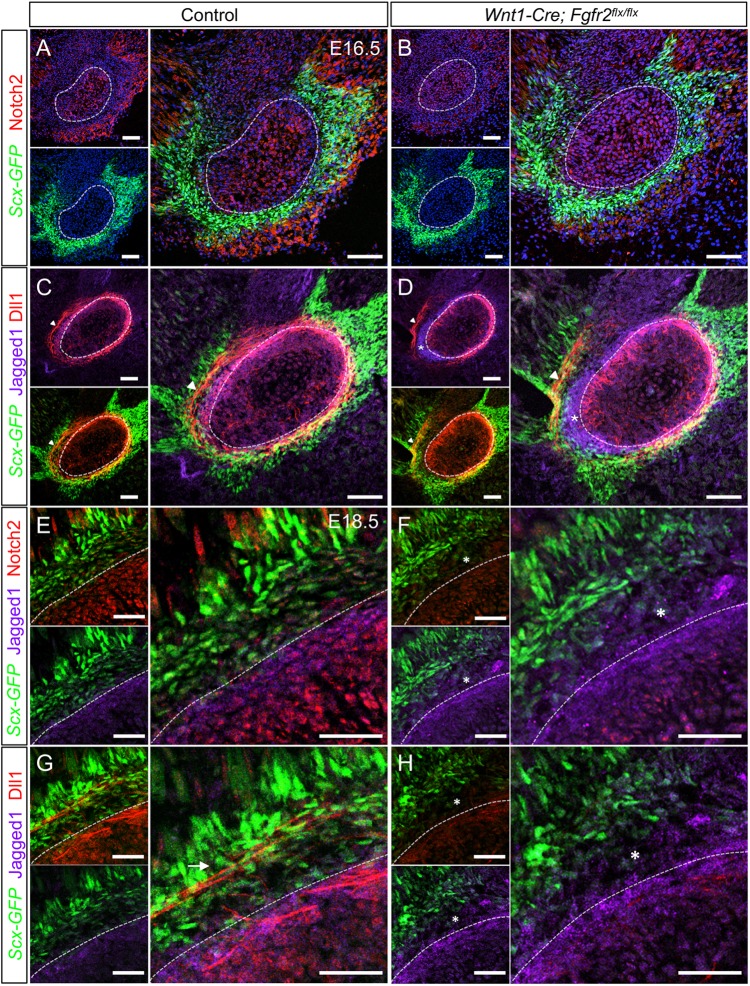
Loss of Fgfr2 alters expression of Notch pathway members at the tendon-bone interface. (A) In control at E16.5, Notch2 is high in the angular cartilage (dashed line), perichondrium and muscular connective tissue, whereas it is low in the tendon. (B) In Wnt1-Cre; Fgfr2flx/flx embryos, Notch2 is reduced throughout the angular process and nearby tissues. (C) Dll1 and Jag1 (Jagged1) overlap in the perichondrial region of the control. Dll1 is also localized to cellular extensions in the Scx-GFP+ tendon (arrowhead). (D) In the Wnt1-Cre; Fgfr2flx/flx angular process, Dll1 and Jag1 occupy distinct domains in the perichondrium (asterisk). The cellular extensions expressing Dll1 in the tendon were displaced from the perichondrial surface (arrowhead). (E) In the control angular process at E18.5, Notch2 and Jag1 overlap in the perichondrial region. Notch2 is lower in the developing tendon-bone attachment populated by Scx+ and Scx− cells. Notch2 is also detected in the muscular connective tissue near the myotendinous junction. (F) In the Wnt1-Cre; Fgfr2flx/flx angular process, Notch2 is reduced overall and Jag1 is expanded in the tendon-bone attachment, which is primary populated by Scx− cells (asterisk). (G) In the control angular process, Dll1 and Jag1 are co-expressed in the perichondrium. Dll1 is also expressed in cellular extensions in Scx+ cells of the tendon (arrow). (H) In the mutant angular process, Dll1 is reduced throughout (asterisk). Smaller panels show individual fluorescent channels with DAPI. n=3 littermate pairs for each. Scale bars: 100 µm in A-D; 50 µm in E-H.
We next looked for expression changes in the Notch2 ligands jagged 1 (Jag1) and Dll1. In the control angular process at E16.5, Jag1 was expressed in the perichondrial region overlapping Fgfr2 and Dll1 (n=3) (Fig. 9C) (Fig. S6B). Dll1 was also identified in cellular extensions of Scx+ cells within the tendon, similar to the pattern seen for Fgf2 (Fig. 9C, arrowhead; Fig. 8C). In the Wnt1-Cre; Fgfr2flx/flx angular process, the overlapping domain of Dll1 and Jag1 was disrupted. Jag1 was expressed in an ectopic domain outside the perichondrium in a region largely populated by Scx− cells that overlapped with the displaced Fgfr2 knockout cells (n=3) (Fig. 9D, asterisk) (Fig. S6C, asterisk). Dll1 was maintained in the perichondrium; however, cellular extensions expressing Dll1 in the tendon were displaced from the perichondrial surface (Fig. 9D, arrowhead). This is consistent with our finding that tendon is displaced from the surface of the angular process in Wnt1-Cre; Fgfr2flx/flx embryos (Fig. 7B). Taken together, these findings demonstrate that loss of Fgfr2 signaling alters the pattern of Notch2-Jag1-Dll1 signaling in the tendon-bone interface during the differentiation of Scx+/Sox9+ cells.
We next examined expression of Notch2, Jag1 and Dll1 in the angular process at E18.5 during tendon-bone attachment unit formation. In the perichondrial region of the control angular process, Notch2, Jag1 and Dll1 were overlapping (n=3) (Fig. 9E,G). In the tendon-bone attachment, which contained a mixture of Scx+ and Scx− cells, Notch2 was expressed along with Dll1+ cellular extensions that appeared to extend from the tendon and perichondrium (n=3) (Fig. 9E,G, arrow). Notch2 was also detected in the muscular connective tissue near the myotendinous junction. Conversely, the perichondrial region and developing tendon-bone attachment of the Wnt1-Cre; Fgfr2flx/flx angular process, which was largely populated by Scx− cells, showed reduced Notch2 and Dll1 and an expanded domain of Jag1 (n=3) (Fig. 9F,H, asterisks). Likewise, Dll1 was markedly reduced within the tendon. These data suggest that, in the developing tendon-bone attachment of the angular process, Notch2-Dll1 signaling is crucial for establishing a mixed region composed of Scx+ and Sox9+ cells.
Notch signaling regulates development of tendon-bone attachment units in the mandible
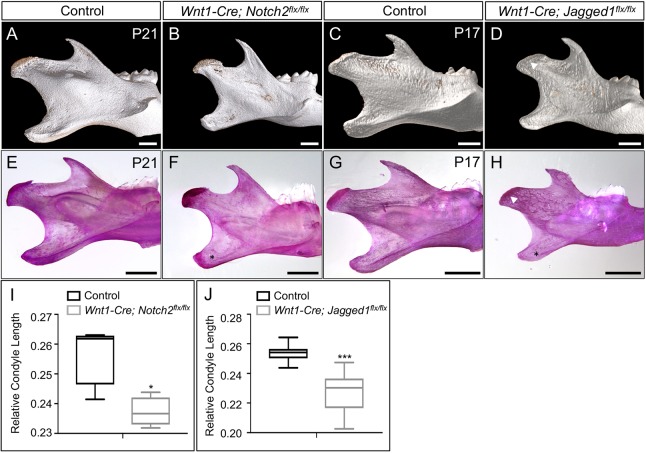
Our data in Wnt1-Cre; Fgfr2flx/flx mice suggested that Notch signaling regulates development of tendon-bone attachment units in the mandible. To test this, we conditionally deleted Notch2 and Jag1 using Wnt1-Cre driver and examined the condyle and angular process. μCT and morphometric analyses of Wnt1-Cre; Notch2flx/flx mice at P21 and Wnt1-Cre; Jagged1flx/flx mice at P17 showed that the condyle and angular process were truncated (P<0.05 and P<0.001) and dysmorphic compared with controls (n=4 same-sex littermate pairs for each) (Fig. 10A-D,I,J) (Fig. S7A-B). In addition, the articular surface on the condyle of Jag1 mutant mice was largely missing (Fig. 10D, arrowhead). Whole-mount skeletal preparations confirmed these findings and revealed that the eminence ridge for the masseteric tendon, which stretches along the surface of the angular process, was largely missing in Notch2 and Jag1 mutant mandibles (Fig. 10E-H, asterisks). Together these results show a functional role for Notch2 and Jag1 in mandibular eminence development.
Fig. 10.
Notch signaling is required for normal development of the tendon-bone attachment unit. (A,B) μCT analyses at P21 show that Wnt1-Cre; Notch2flx/flx mandibles have smaller and morphologically irregular condyle and angular process compared with controls. (C,D) Wnt1-Cre; Jagged1flx/flx mandibles at P17 also have a smaller condyle and angular process (arrowhead). (E-H) Whole-mount skeletal preparations from control and Wnt1-Cre; Notch2flx/flx at P21 and Wnt1-Cre; Jagged1flx/flx at P17 reveal that the ridge for the masseter tendon on the angular process is missing (asterisks). (G,H) In the Wnt1-Cre; Jagged1flx/flx mandible, the articular surface of the condyle is missing (arrowhead). (I-J) Morphometric analyses show that the relative condyle length in Wnt1-Cre; Notch2flx/flx (I) and Wnt1-Cre; Jagged1flx/flx (J) mandibles are truncated (n=4 same-sex littermate pairs). *P<0.05, ***P<0.001 (two-tailed t-test). Error bars represent s.e.m. Scale bars: 1 mm.
DISCUSSION
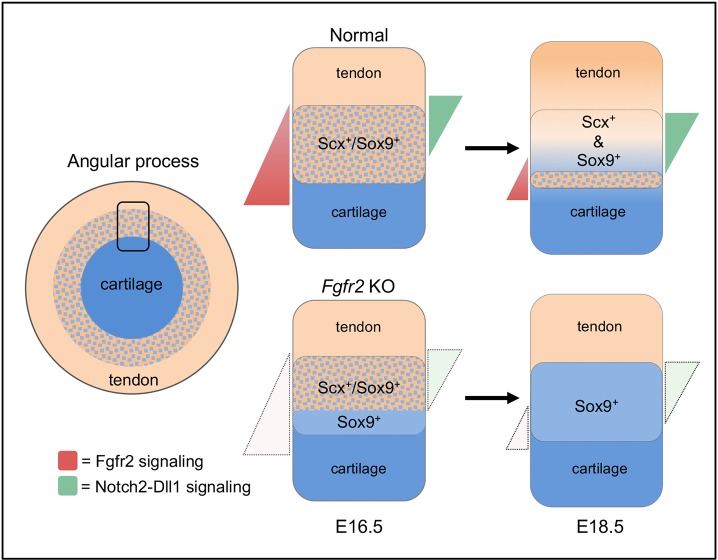
This study shows that Fgfr2 signaling directs development of tendon-bone attachment units in the mandible (Fig. 11). Loss of Fgfr2 signaling reduces Scx expression in Scx+/Sox9+ progenitors and promotes differentiation of Sox9+ chondrocytes over Scx+ tenocytes, subsequently inducing ectopic endochondral bone formation outside the eminence boundary. We find that loss of the mixed region of chondrocytes and tenocytes in the tendon-bone interface of Fgfr2 mutant mandibles is concomitant with decreased Notch2 expression and altered patterns of Jag1 and Dll1 expression. Correspondingly, loss of Notch2 and Jag1 disrupts normal development of the mandibular eminences. By identifying FGF signaling as an upstream regulator of Notch signaling in development of tendon-bone attachment units, this work reveals new insights into the mechanisms that direct development of a zonally organized tissue.
Fig. 11.
Fgfr2 regulates development of tendon-bone attachment in the mandible. Scx+/Sox9+ progenitors populate the tendon-bone interface. High Fgfr2 signaling in the perichondrium promotes low Notch signaling through co-expression of Notch2-Jag1-Dll1 to maintain Scx+/Sox9+ progenitors. In juxta-tendon regions, in which Fgfr2 signaling is low, Notch2-Dll1 signaling is activated and Scx+/Sox9+ progenitors differentiate into a mixed-cell region of Sox9+ chondrocytes and Scx+ tenocytes. In the absence of Fgfr2, Notch2 signaling is reduced and Scx+/Sox9+ cells undergo biased differentiation into Sox9+ chondrocytes.
Our findings demonstrate that Fgfr2 signaling promotes formation of Scx+ cells during tendon-bone attachment unit development. This is consistent with published studies showing that FGF family proteins are sufficient to activate Scx expression and tenocyte cell fate in chick somites and limbs (Brent et al., 2003; Brent and Tabin, 2004; Havis et al., 2016). However, the effect of FGF signaling on tendon development in mice is less clear. Tendon defects have yet to be described in mice that harbor gene mutations in FGF and FGFR family proteins and the outcome of FGF treatment on Scx expression in vitro differs between studies (Havis et al., 2014; Ker et al., 2011). Although FGF-mediated regulation of Scx appears to be context dependent, our results provide genetic evidence that Fgfr2-Fgf2 signaling promotes Scx expression and tenocyte cell fate in Scx+/Sox9+ progenitors. Conditional deletion of Fgfr2 before and after the onset of Scx expression using Wnt-Cre and Scx-Cre, respectively, decreased Scx expression in Scx+/Sox9+ progenitors and disrupted tendon insertions. However, the phenotype resulting from Wnt1-Cre-mediated inactivation of Fgfr2 was more severe, with an increase in Sox9+ single-positive cells appearing at the tendon-bone interface at E15.5 and subsequently more extensive ectopic bone formation in the postnatal period. These results indicate an early role for Fgfr2 signaling in maintaining Scx+/Sox9+ cells. However, it is not yet known whether Fgfr2 is solely responsible for FGF-mediated regulation of Scx+/Sox9+ cells. Fgfr1 and Fgfr3 are restricted to chondroblastic and hypertrophic layers within the postnatal condylar cartilage and Fgfr3 signaling regulates chondrocyte maturation in the condyle (Duplan et al., 2016; Fuentes et al., 2002; Yasuda et al., 2012). Thus, these FGFR family proteins have the potential to regulate eminence formation within the tendon-bone attachment units of the mandible.
We show evidence that Fgfr2-Fgf2 signaling regulates cell fate at the tendon-bone interface by spatially deploying Notch2 signaling. Notch signaling is a key regulator of binary cell fate choice, and the cellular outcomes of Notch-Jagged and Notch-Delta signaling are distinct (Sjoqvist and Andersson, 2019). Notch activation by Jagged induces neighboring cells within a pool of equivalent progenitors to adopt a common cell fate through lateral induction. Notch activation by Delta inhibits neighboring Notch-expressing progenitors from adopting the default cell fate through lateral inhibition. Interactions between Notch and its ligands within the same cell attenuate their activity through cis-inhibition (del Álamo et al., 2011). We provide evidence that regional specific localization of Notch2, Jag1 and Dll1 at the tendon-bone interface determines the pattern of bipotent Scx+/Sox9+ progenitor differentiation. In the perichondrial region at E16.5, where FGF signaling is high, Notch2-Jag1-Dll1 expression in Scx+/Sox9+ progenitors is expected to result in low Notch signaling through cis-inhibition. As the mandibular cartilages undergo appositional growth, we predict that the Scx+/Sox9+ progenitors experiencing less FGF signaling move into a tendon-adjacent domain permissive for high Notch signaling. Indeed, at E18.5, Scx+/Sox9+ cells remaining in a perichondrial-adjacent domain expressed Notch2-Jag1-Dll1, whereas the mixed-cell region of Scx+ and Sox9+ cells localized to a tendon-adjacent domain expressed Notch2-Dll1. Therefore, we hypothesize that Notch2-Dll1 signaling in Scx+/Sox9+ progenitors induces formation of this mixed cell region through lateral inhibition. Our model is further supported by our findings in Wnt1-Cre; Fgfr2flx/flx mice. At E16.5, an increase in Sox9+ cells in the Fgfr2 mutant coincides with decreased Notch2 and mutually exclusive domains of Jag1 and Dll1. At E18.5, early depletion of Scx+/Sox9+ progenitors is linked to loss of Notch2-Jag1-Dll1 in the perichondrium and biased differentiation of Sox9+ chondrocytes is linked to reduced Notch2-Dll1 in the tendon-bone attachment. Furthermore, it has been shown in other developmental processes that FGF-dependent Notch signaling establishes cell fate as a function of the position of the cell within a gradient of FGF signaling (Akai et al., 2005).
In the limb, specification of Scx+/Sox9+ progenitors requires TGFβ signaling (Blitz et al., 2013). Although the necessity of TGFβ signaling in Scx+/Sox9+ progenitor specification has yet to be determined in the mandible, knockout of Tgfbr2 prevents formation of the secondary cartilages in the condyle and angular process (Oka et al., 2008). We found that loss of Fgfr2 results in a more discrete phenotype due to abnormal growth and differentiation of the secondary cartilages. Although Fgfr2 is dispensable for initial specification of Scx+/Sox9+ progenitors, our data supports a role for the receptor in Scx+/Sox9+ progenitor maintenance and differentiation in the mandible. In the limb, differentiation of Scx+/Sox9+ progenitors into chondrocytes requires BMP signaling (Blitz et al., 2013, 2009). Opposing gradients of BMP and FGF signaling regulate cell fate at developmental borders, often through cross-inhibition. As antagonistic interactions between FGF and BMP signaling regulate chondrogenesis (Yoon et al., 2006), it will be important to determine whether ectopic chondrogenesis in tendon-bone attachment units of Fgfr2 mutant mice is in part due to deregulation of BMP signaling. As the embryonic tendon-bone attachment matures in the postnatal limb, Gli1+ cells derived from the Sox9-lineage contribute to fibrocartilage and mineralized fibrocartilage (Felsenthal et al., 2018). Although a relationship between Fgfr2 and hedgehog signaling in the tendon-bone attachment has yet to be determined, it will also be important to investigate how an increase in Sox9+ cells in the Fgfr2 mutant alters development of their Gli1+ derivatives.
MATERIALS AND METHODS
Mice
The Scx-Cre line used has previously been described (Blitz et al., 2009). To conditionally knockout Fgfr2 in NCCs, Fgfr2flx/flx (JAX 007569, The Jackson Laboratory) mice were crossed with the Wnt1-Cre2 driver (JAX 022137, The Jackson Laboratory) or Scx-Cre. The Fgfr2flx/flx and Wnt1-Cre2 lines have previously been described (Lewis et al., 2013; Yu et al., 2003). Scx-GFP was used to mark Scx+/Sox9+ progenitors, as well as tendon and ligament (Pryce et al., 2007). To conditionally knockout Notch2 in NCCs, the Wnt1-Cre1 driver was crossed with Notch2flx/flx (JAX 010525, The Jackson Laboratory) mice. The Notch2flx/flx line has previously been described (Kiernan et al., 2006). The Ai9 allele (JAX 007909, The Jackson Laboratory) was used as a lineage marker for those tissues targeted by Wnt1-Cre2 and Scx-Cre (Madisen et al., 2010). Scx−/− mice have previously been described (Murchison et al., 2007). Embryonic samples were collected from timed pregnant females. Postnatal samples were staged according to the date of birth. All experimental protocols were approved by the University of Southern California Institutional Animal Care and Use Committee.
Immunofluorescent analysis
Embryos and postnatal samples were fixed in 4% paraformaldehyde (PFA) for 15 min to 3 h, depending on the sample size and age. Postnatal tissues were then decalcified with 10% EDTA (pH 7.4) for 1-3 weeks at 4°C. Embryonic and postnatal tissues were equilibrated in 30% sucrose/PBS at 4°C, embedded in optimal cutting temperature (O.C.T.) compound (Electron Microscopy Sciences) and cryosectioned in the coronal plane at 8 μm. Frozen sections were then washed with PBST (1× PBS with 0.1% Triton X-100) and blocked with 10% donkey or goat serum (Sigma-Aldrich) for 1 h at room temperature. When mouse primary antibodies were used, the sections were pre-incubated with Anti-fab fragment for 1 h at room temperature. All sections were incubated with primary antibodies overnight at 4°C. See Table S1 for antibody vendor information and dilutions. Sections were washed with PBST and incubated with Alexa Fluor secondary antibodies (see Table S3) at 1:500/PBST for 1 h at room temperature, washed with PBST and mounted with Vectashield containing DAPI (Vector Labs). Slides were imaged on a Leica TCS SP5/8 confocal system. These experiments were performed on at least three biological replicates, which we defined as three littermate pairs (control and mutant) derived from three different litters.
Skeletal preparation
Samples were skinned, eviscerated and fixed in 95% ethanol for 3-5 days. Fixed samples were incubated in Alcian Blue stain [0.15 mg/ml Alcian Blue 8GX (Sigma-Aldrich) in 80% ethanol and 20% glacial acetic acid] overnight and de-stained in 95% ethanol for 2 days. Tissue samples were then cleared with 0.5-1% KOH [w/v] for 1-5 days, depending on size, and incubated in Alizarin Red solution [0.02 mg/ml Alizarin Red S (Sigma-Aldrich) in 0.5-1% KOH] for an additional 1-5 days. Stained specimens were equilibrated in 75% glycerol for imaging. These experiments were performed on at least three biological replicates, which we defined as three littermate pairs (control and mutant) derived from three different litters.
Histology
For histological staining, samples were fixed in 4% PFA for 30 min to 3 h, decalcified with BBC Biochemical Rapid Cal Immuno (Thermo Fisher Scientific) overnight at room temperature, dehydrated in an ethanol series, equilibrated in Citrisolv, embedded in paraffin and sectioned in the coronal plane at 8 μM. Connective tissues were discriminated using Hall-Brunt quadruple stain (HBQ) (Hall, 1986). For undecalcified specimens, skulls were carefully cleaned of soft tissues, fixed in 70% ethanol at room temperature, dehydrated in an ethanol series, cleared with xylene and then embedded in methyl methacrylate. Coronal sections were cut at a thickness of 5 μm using a microtome (Jung Supercut, Reichert-Jung) and stained with Toluidine Blue (pH 6.4) and von Kossa. These experiments were performed on at least three biological replicates, which we defined as three littermate pairs (control and mutant) derived from three different litters.
In situ hybridization
Transcripts of Scx and Sox9 were detected using RNAscope Fluorescent Multiplex Assay (ACD) as per the manufacturer's instructions at E15.5. Briefly, slides with paraffin sections were baked on a hot plate for 30 min at 43°C. Slides were then deparaffinized using CitriSolv, dehydrated in 100% EtOH and allowed to completely air dry. Endogenous peroxidase activity was quenched using the hydrogen peroxide provided within the kit, slides were washed in deionized water. Antigen retrieval was performed using the provided Target Retrieval Reagent for 20 min in an Oster steamer heated to 99°C, followed by dehydration in 100% EtOH and complete air drying. The slides were treated with ACD Protease Plus in a humidified slide chamber for 20 min at 40°C. Probe hybridization of the Scx (ACD, 439981) and Sox9 (ACD, 401051-C2) probes was also performed in a 40°C humidified slide chamber for 2 h. Slides were then stored in 5× SSC solution overnight. Amplification steps were performed as prescribed by manufacturer and signal development for channels one and two were carried out using TSA Plus fluorophores Cyanine 3 (PerkinElmer, NEL744E001KT) and Cyanine 5 (PerkinElmer, NEL705A001KT) diluted 1:750 in the ACD-provided TSA buffer. Slides were then counterstained using Vectashield mounting medium with DAPI and imaged no later than 16 h after completion of protocol using confocal microscopy. All steps, other than those recommended by the manufacturer to complete in a humidified slide chamber at 40°C, were carried out using plastic five-slide capacity mailers at room temperature. Slides were images using the Leica TCS SP5/8 confocal system confocal microscope and mean fluorescent intensity of the Scx+/Sox9+ region was quantified from 18 regions of interest per section using Leica LAS X software. P-values were calculated using two-tailed t-test assuming unequal variance. These experiments were performed on three biological replicates, which we defined as three littermate pairs (control and mutant) derived from two different litters.
Micro-computed tomography
All μCT scans were performed by the University of Southern California Molecular Imaging Center using a μCT50 (Scanco Medical). Samples were rotated 360° and X-ray settings were standardized to 90 kV and 155 µA with an exposure time of 0.5 s per frame to yield a nominal resolution of 20 μM. A 0.5-mm-thick aluminum filter was employed to minimize beam-hardening artifacts. Morphometric analysis was performed using the Amira 6.2 and VG Studio MAX 3.0 software packages. Isosurface renderings with equal threshold were measured using the 3D measuring tool. All jaw measurements and landmarks were measured as previously described (Guerreiro et al., 2013). These experiments were performed on at least three biological replicates, which we defined as three same-sex littermate pairs (control and mutant) derived from three different litters. Statistical significance was determined using unpaired two-tailed t-tests.
RNA isolation and gene expression analysis of the angular process
The angular process was dissected at E16.5 and RNA isolated using the Qiagen RNeasy Mini Prep Plus Kit according to the manufacturer's instructions. The RNA concentration was measured and 50 ng of total RNA was used for amplification using Kapa Sybr Fast one-step quantitative real-time PCR reagents per the manufacturer's instructions using the primers listed in Table S2. Expression analysis was monitored on a Bio-Rad CFX96 Real-Time System based on relative gene expression using ΔCT calculation. Samples were normalized to actin and statistical significance was determined using unpaired two-tailed t-tests.
Western blotting
Frontal and parietal bones were surgically dissected from P5 pups and incubated in NP40 lysis buffer [50 mM Tris (pH 7.4), 250 mM NaCl, 5 mM EDTA, 50 mM NaF, 1 mM Na3VO4, and 1% Nonidet P40 (NP40)] containing protease inhibitors (Roche) and 1 mM phenylmethylsulfonyl fluoride, and were subjected to three freeze/thaw cycles. Lysates were incubated on ice for 30 min and protein was measured using a Bradford Protein Assay (Bio-Rad). Normalized protein concentrations were resolved on 8% SDS-PAGE gel, transferred to PVDF membrane and probed with anti-Fgfr2 (Santa Cruz, sc-122, 1:1000) or anti-α-tubulin (Sigma, T6793, 1:40,000) overnight at 4°C. Immunoreactivity was detected using a Phototope-HRP western blot detection system according to the manufacturer's instructions (Cell Signaling Technology) (n=3).
Supplementary Material
Acknowledgements
We thank Cynthia Neben, Diana Rigueur, Joanna Salva and Francesca Mariani for insightful discussions. We also thank Bridget Samuels for critically reviewing the manuscript. We thank Robert Maxson for supplying the Notch2 and Jag1 conditional knockout line and the University of Southern California Molecular Imaging Center for their help with small animal CT imaging. We also thank Jorge Martin, Deborah Krakow and Renata Pereira (UCLA Bone Histomorphometric Core Laboratory) for preparation and staining of the histology sections.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: A.E.M.; Methodology: A.E.M., R.R.R.; Validation: A.E.M., R.R.R., C.T.T.; Formal analysis: A.E.M., R.R.R., L.B., C.S.T., C.T.T.; Investigation: A.E.M., R.R.R., L.B.; Resources: C.S.T., D.P., R.S.; Data curation: A.E.M., R.R.R.; Writing - original draft: A.E.M., R.R.R.; Writing - review & editing: A.E.M., R.R.R., L.B., R.S., C.T.T.; Visualization: A.E.M., R.R.R., L.B.; Project administration: A.E.M.; Funding acquisition: A.E.M.
Funding
This work was supported by the National Institutes of Health [1R01DE025222 to A.E.M; Diversity Supplement R01 DE025222-01S1 and T90DE021982 to R.R.R.; T90DE021982 to L.B.], March of Dimes Foundation [36-FY15-233 to A.E.M.] and Shriners Hospitals for Children [85410-POR-14 to R.S.]. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.170241.supplemental
References
- Akai J., Halley P. A. and Storey K. G. (2005). FGF-dependent Notch signaling maintains the spinal cord stem zone. Genes Dev. 19, 2877-2887. 10.1101/gad.357705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H., Kim J.-E., Nakashima K., Balmes G., Iwai N., Deng J. M., Zhang Z., Martin J. F., Behringer R. R., Nakamura T. et al. (2005). Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc. Natl. Acad. Sci. USA 102, 14665 10.1073/pnas.0504750102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthwal N. and Tucker A. (2012). From Clone to Bone: The Synergy of Morphological and Molecular Tools in Palaeobiology. Cambridge, United Kingdom: Cambridge University Press. [Google Scholar]
- Anthwal N., Chai Y. and Tucker A. S. (2008). The role of transforming growth factor-β signalling in the patterning of the proximal processes of the murine dentary. Dev. Dyn. 237, 1604-1613. 10.1002/dvdy.21567 [DOI] [PubMed] [Google Scholar]
- Baverstock H., Jeffery N. S. and Cobb S. N. (2013). The morphology of the mouse masticatory musculature. J. Anat. 223, 46-60. 10.1111/joa.12059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin M., Toumi H., Ralphs J. R., Bydder G., Best T. M. and Milz S. (2006). Where tendons and ligaments meet bone: attachment sites (‘entheses’) in relation to exercise and/or mechanical load. J. Anat. 208, 471-490. 10.1111/j.1469-7580.2006.00540.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz E., Viukov S., Sharir A., Shwartz Y., Galloway J. L., Pryce B. A., Johnson R. L., Tabin C. J., Schweitzer R. and Zelzer E. (2009). Bone ridge patterning during musculoskeletal assembly is mediated through SCX regulation of Bmp4 at the tendon-skeleton junction. Dev. Cell 17, 861-873. 10.1016/j.devcel.2009.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz E., Sharir A., Akiyama H. and Zelzer E. (2013). Tendon-bone attachment unit is formed modularly by a distinct pool of Scx- and Sox9-positive progenitors. Development 140, 2680-2690. 10.1242/dev.093906 [DOI] [PubMed] [Google Scholar]
- Brent A. E. and Tabin C. J. (2004). FGF acts directly on the somitic tendon progenitors through the Ets transcription factors Pea3 and Erm to regulate scleraxis expression. Development 131, 3885-3896. 10.1242/dev.01275 [DOI] [PubMed] [Google Scholar]
- Brent A. E., Schweitzer R. and Tabin C. J. (2003). A somitic compartment of tendon progenitors. Cell 113, 235-248. 10.1016/S0092-8674(03)00268-X [DOI] [PubMed] [Google Scholar]
- Chen J., Utreja A., Kalajzic Z., Sobue T., Rowe D. and Wadhwa S. (2012). Isolation and characterization of murine mandibular condylar cartilage cell populations. Cells Tissues Organs 195, 232-243. 10.1159/000325148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Álamo D., Rouault H. and Schweisguth F. (2011). Mechanism and significance of cis-inhibition in Notch signalling. Curr. Biol. 21, R40-R47. 10.1016/j.cub.2010.10.034 [DOI] [PubMed] [Google Scholar]
- Duplan M., Komla-Ebri D., Heuzé Y., Estibals V., Gaudas E., Kaci N., Benoist-Lasselin C., Zerah M., Kramer I., Kneissel M. et al. (2016). Meckel's and condylar cartilages anomalies in achondroplasia result in defective development and growth of the mandible. Hum. Mol. Genet. 25, 2997-3010. 10.1093/hmg/ddw153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyment N. A., Breidenbach A. P., Schwartz A. G., Russell R. P., Aschbacher-Smith L., Liu H., Hagiwara Y., Jiang R., Thomopoulos S., Butler D. L. et al. (2015). Gdf5 progenitors give rise to fibrocartilage cells that mineralize via hedgehog signaling to form the zonal enthesis. Dev. Biol. 405, 96-107. 10.1016/j.ydbio.2015.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eswarakumar V. P., Monsonego-Ornan E., Pines M., Antonopoulou I., Morriss-Kay G. M. and Lonai P. (2002). The IIIc alternative of Fgfr2 is a positive regulator of bone formation. Development 129, 3783-3793. [DOI] [PubMed] [Google Scholar]
- Eswarakumar V. P., Horowitz M. C., Locklin R., Morriss-Kay G. M. and Lonai P. (2004). A gain-of-function mutation of Fgfr2c demonstrates the roles of this receptor variant in osteogenesis. Proc. Natl. Acad. Sci. USA 101, 12555-12560. 10.1073/pnas.0405031101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenthal N., Rubin S., Stern T., Krief S., Pal D., Pryce B. A., Schweitzer R. and Zelzer E. (2018). Development of migrating tendon-bone attachments involves replacement of progenitor populations. Development 145, dev165381 10.1242/dev.165381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes M. A., Opperman L. A., Bellinger L. L., Carlson D. S. and Hinton R. J. (2002). Regulation of cell proliferation in rat mandibular condylar cartilage in explant culture by insulin-like growth factor-1 and fibroblast growth factor-2. Arch. Oral Biol. 47, 643-654. 10.1016/S0003-9969(02)00052-3 [DOI] [PubMed] [Google Scholar]
- Guerreiro F. d. S., Diniz P., Carvalho P. E. G., Ferreira E. C., Avancini S. R. P. and Ferreira-Santos R. I. (2013). Effects of masticatory hypofunction on mandibular morphology, mineral density and basal bone area. Braz. J. Oral Sci. 12, 205-211. 10.1590/S1677-32252013000300010 [DOI] [Google Scholar]
- Hall B. K. (1986). The role of movement and tissue interactions in the development and growth of bone and secondary cartilage in the clavicle of the embryonic chick. J. Embryol. Exp. Morphol. 93, 133-152. [PubMed] [Google Scholar]
- Hall B. K. (2015). Bones and cartilage (Second Edition). In Bones and Cartilage, 2nd edn (ed. Hall B. K.), pp. 15-16. San Diego: Academic Press. [Google Scholar]
- Havis E., Bonnin M.-A., Olivera-Martinez I., Nazaret N., Ruggiu M., Weibel J., Durand C., Guerquin M.-J., Bonod-Bidaud C., Ruggiero F. et al. (2014). Transcriptomic analysis of mouse limb tendon cells during development. Development 141, 3683-3696. 10.1242/dev.108654 [DOI] [PubMed] [Google Scholar]
- Havis E., Bonnin M.-A., Esteves de Lima J., Charvet B., Milet C. and Duprez D. (2016). TGFβ and FGF promote tendon progenitor fate and act downstream of muscle contraction to regulate tendon differentiation during chick limb development. Development 143, 3839-3851. 10.1242/dev.136242 [DOI] [PubMed] [Google Scholar]
- He Y., Zhang M., Huang A. Y., Cui Y., Bai D. and Warman M. L. (2017). Confocal imaging of mouse mandibular condyle cartilage. Sci. Rep. 7, 43848 10.1038/srep43848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems T. and Tillmann B. (2000). Tendon entheses of the human masticatory muscles. Anat. Embryol. 202, 201-208. 10.1007/s004290000107 [DOI] [PubMed] [Google Scholar]
- Hinton R. J. and Carlson D. S. (2005). Regulation of growth in mandibular condylar cartilage. Semin. Orthod. 11, 209-218. 10.1053/j.sodo.2005.07.005 [DOI] [Google Scholar]
- Ker E. D., Chu B., Phillippi J. A., Gharaibeh B., Huard J., Weiss L. E. and Campbell P. G. (2011). Engineering spatial control of multiple differentiation fates within a stem cell population. Biomaterials 32, 3413-3422. 10.1016/j.biomaterials.2011.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan A. E., Xu J. and Gridley T. (2006). The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2, e4 10.1371/journal.pgen.0020004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.-H., Pryce B. A., Schweitzer R., Ryder M. I. and Ho S. P. (2015). Differentiating zones at periodontal ligament-bone and periodontal ligament-cementum entheses. J. Periodontal Res. 50, 870-880. 10.1111/jre.12281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A. E., Vasudevan H. N., O'Neill A. K., Soriano P. and Bush J. O. (2013). The widely used Wnt1-Cre transgene causes developmental phenotypes by ectopic activation of Wnt signaling. Dev. Biol. 379, 229-234. 10.1016/j.ydbio.2013.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H. H. and Thomopoulos S. (2013). Functional attachment of soft tissues to bone: development, healing, and tissue engineering. Annu. Rev. Biomed. Eng. 15, 201-226. 10.1146/annurev-bioeng-071910-124656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L., Zwingman T. A., Sunkin S. M., Oh S. W., Zariwala H. A., Gu H., Ng L. L., Palmiter R. D., Hawrylycz M. J., Jones A. R. et al. (2010). A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133-140. 10.1038/nn.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka T., Ahlberg P. E., Kessaris N., Iannarelli P., Dennehy U., Richardson W. D., McMahon A. P. and Koentges G. (2005). Neural crest origins of the neck and shoulder. Nature 436, 347 10.1038/nature03837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill A. E., Sarukhanov A., Krejci P., Idoni B., Camacho N., Estrada K. D., Lyons K. M., Deixler H., Robinson H., Chitayat D. et al. (2012). Bent bone dysplasia-FGFR2 type, a distinct skeletal disorder, has deficient canonical FGF signaling. Am. J. Hum. Genet. 90, 550-557. 10.1016/j.ajhg.2012.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millimaki B. B., Sweet E. M., Dhason M. S. and Riley B. B. (2007). Zebrafish atoh1 genes: classic proneural activity in the inner ear and regulation by Fgf and Notch. Development 134, 295-305. 10.1242/dev.02734 [DOI] [PubMed] [Google Scholar]
- Murchison N. D., Price B. A., Conner D. A., Keene D. R., Olson E. N., Tabin C. J. and Schweitzer R. (2007). Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development 134, 2697-2708. 10.1242/dev.001933 [DOI] [PubMed] [Google Scholar]
- Ogawa T., Shimokawa H., Fukada K., Suzuki S., Shibata S., Ohya K. and Kuroda T. (2003). Localization and inhibitory effect of basic fibroblast growth factor on chondrogenesis in cultured mouse mandibular condyle. J. Bone Miner. Metab. 21, 145-153. 10.1007/s007740300023 [DOI] [PubMed] [Google Scholar]
- Oka K., Oka S., Hosokawa R., Bringas P. Jr, Brockhoff H. C. II, Nonaka K. and Chai Y. (2008). TGF-beta mediated Dlx5 signaling plays a crucial role in osteo-chondroprogenitor cell lineage determination during mandible development. Dev. Biol. 321, 303-309. 10.1016/j.ydbio.2008.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz D. M. and Marie P. J. (2015). Fibroblast growth factor signaling in skeletal development and disease. Genes Dev. 29, 1463-1486. 10.1101/gad.266551.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce B. A., Brent A. E., Murchison N. D., Tabin C. J. and Schweitzer R. (2007). Generation of transgenic tendon reporters, ScxGFP and ScxAP, using regulatory elements of the scleraxis gene. Dev. Dyn. 236, 1677-1682. 10.1002/dvdy.21179 [DOI] [PubMed] [Google Scholar]
- Purcell P., Jheon A., Vivero M. P., Rahimi H., Joo A. and Klein O. D. (2012). Spry1 and Spry2 are essential for development of the temporomandibular joint. J. Dent. Res. 91, 387-393. 10.1177/0022034512438401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rot-Nikcevic I., Downing K. J., Hall B. K. and Kablar B. (2007). Development of the mouse mandibles and clavicles in the absence of skeletal myogenesis. Histol. Histopathol. 22, 51-60. 10.14670/HH-22.51 [DOI] [PubMed] [Google Scholar]
- Schwartz A. G., Long F. and Thomopoulos S. (2015). Enthesis fibrocartilage cells originate from a population of Hedgehog-responsive cells modulated by the loading environment. Development 142, 196 10.1242/dev.112714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibukawa Y., Young B., Wu C., Yamada S., Long F., Pacifici M. and Koyama E. (2007). Temporomandibular joint formation and condyle growth require Indian hedgehog signaling. Dev. Dyn. 236, 426-434. 10.1002/dvdy.21036 [DOI] [PubMed] [Google Scholar]
- Sjoqvist M. and Andersson E. R. (2019). Do as I say, Not(ch) as I do: lateral control of cell fate. Dev. Biol. 447, 58-70. 10.1016/j.ydbio.2017.09.032 [DOI] [PubMed] [Google Scholar]
- Soeda T., Deng J. M., de Crombrugghe B., Behringer R. R., Nakamura T. and Akiyama H. (2010). Sox9-expressing precursors are the cellular origin of the cruciate ligament of the knee joint and the limb tendons. Genesis 48, 635-644. 10.1002/dvg.20667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solem R. C., Eames B. F., Tokita M. and Schneider R. A. (2011). Mesenchymal and mechanical mechanisms of secondary cartilage induction. Dev. Biol. 356, 28-39. 10.1016/j.ydbio.2011.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y., Takimoto A., Akiyama H., Kist R., Scherer G., Nakamura T., Hiraki Y. and Shukunami C. (2013). Scx+/Sox9+ progenitors contribute to the establishment of the junction between cartilage and tendon/ligament. Development 140, 2280-2288. 10.1242/dev.096354 [DOI] [PubMed] [Google Scholar]
- Thomopoulos S., Hattersley G., Rosen V., Mertens M., Galatz L., Williams G. R. and Soslowsky L. J. (2002). The localized expression of extracellular matrix components in healing tendon insertion sites: an in situ hybridization study. J. Orthop. Res. 20, 454-463. 10.1016/S0736-0266(01)00144-9 [DOI] [PubMed] [Google Scholar]
- Tummers M. and Thesleff I. (2003). Root or crown: a developmental choice orchestrated by the differential regulation of the epithelial stem cell niche in the tooth of two rodent species. Development 130, 1049-1057. 10.1242/dev.00332 [DOI] [PubMed] [Google Scholar]
- Wahl M. B., Deng C., Lewandoski M. and Pourquié O. (2007). FGF signaling acts upstream of the NOTCH and WNT signaling pathways to control segmentation clock oscillations in mouse somitogenesis. Development 134, 4033-4041. 10.1242/dev.009167 [DOI] [PubMed] [Google Scholar]
- Woronowicz K. C., Gline S. E., Herfat S. T., Fields A. J. and Schneider R. A. (2018). FGF and TGFβ signaling link form and function during jaw development and evolution. Dev. Biol. 444, S219-S236. 10.1016/j.ydbio.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T., Nah H. D., Laurita J., Kinumatsu T., Shibukawa Y., Shibutani T., Minugh-Purvis N., Pacifici M. and Koyama E. (2012). Muenke syndrome mutation, FgfR3P244R, causes TMJ defects. J. Dent. Res. 91, 683-689. 10.1177/0022034512449170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon B. S., Pogue R., Ovchinnikov D. A., Yoshii I., Mishina Y., Behringer R. R. and Lyons K. M. (2006). BMPs regulate multiple aspects of growth-plate chondrogenesis through opposing actions on FGF pathways. Development 133, 4667-4678. 10.1242/dev.02680 [DOI] [PubMed] [Google Scholar]
- Yu K., Xu J., Liu Z., Sosic D., Shao J., Olson E. N., Towler D. A. and Ornitz D. M. (2003). Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development 130, 3063-3074. 10.1242/dev.00491 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.