Figure 1:
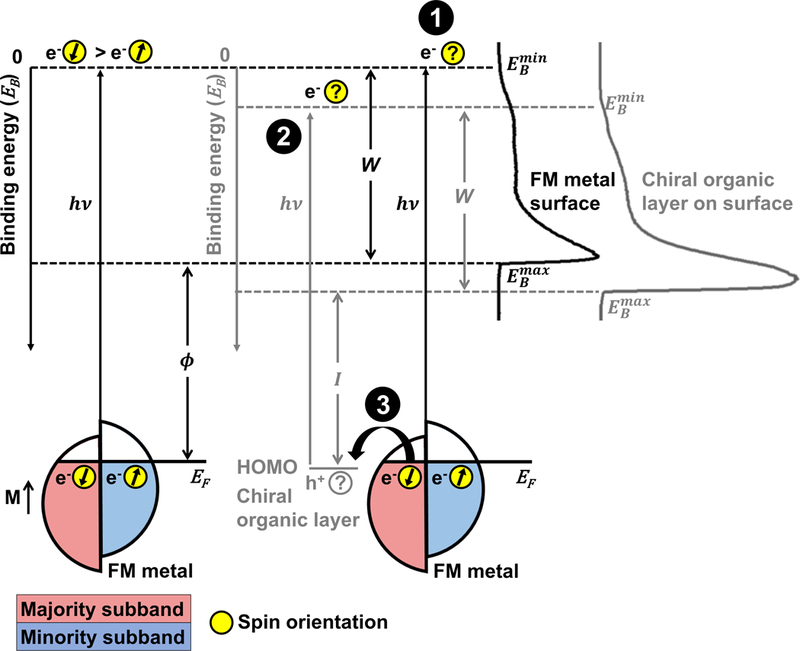
Experimental schematic of ultraviolet photoelectron spectroscopy (UPS) of ferromagnetic multilayer (FM) metal surfaces. Spin-selective processes due to the chiral-induced spin selectivity effect may include filtering of photoelectrons originating from the metal by adsorbed chiral species (1), spin-polarized photoemission by ionization of chiral films (2), or filtering of conduction electrons supplied by the metal to fill holes left in the valence orbitals of the organic films, represented schematically as the highest occupied molecular orbitals (HOMO) (3). The right side of the image depicts representative ultraviolet photoelectron spectra from bare FM surfaces (black) and surfaces coated with chiral organic films (grey). The large peaks at high binding energies correspond to the collection of secondary electrons that scatter, losing energy. The spin polarization of photoelectrons emitted from bare FM surfaces by photons with energy reflects the polarization within the metal dictated by the magnetization orientation, M. The Fermi edge and minimum binding energy, , of the metal is at 0 eV, and the secondary electron cutoff of electrons with maximum binding energy is . When functionalized with organic chiral layers, photoelectrons are collected from both the metal surface as well as the organic material due to ionization. The valence band edge of the spectra, , and the secondary electron cutoff, , represent the highest and lowest kinetic energies of collected photoelectrons, respectively. The minimum energy required to remove an electron from the surface is the work function, . The spectral width, , is used to calculate the photoionization energy, , of the organic films.
