Soil water availability determines the feedback of ecosystem carbon cycle to climate warming.
Abstract
It has been well established by field experiments that warming stimulates either net ecosystem carbon uptake or release, leading to negative or positive carbon cycle–climate change feedback, respectively. This variation in carbon-climate feedback has been partially attributed to water availability. However, it remains unclear under what conditions water availability enhances or weakens carbon-climate feedback or even changes its direction. Combining a field experiment with a global synthesis, we show that warming stimulates net carbon uptake (negative feedback) under wet conditions, but depresses it (positive feedback) under very dry conditions. This switch in carbon-climate feedback direction arises mainly from scaling effects of warming-induced decreases in soil water content on net ecosystem productivity. This water scaling of warming effects offers generalizable mechanisms not only to help explain varying magnitudes and directions of observed carbon-climate feedback but also to improve model prediction of ecosystem carbon dynamics in response to climate change.
INTRODUCTION
Whether climate warming would stimulate additional carbon release from terrestrial ecosystems to accelerate climate change (i.e., the carbon-climate feedback) is among the most uncertain processes for predicting future climate warming. The majority of modeling studies suggests that feedback of terrestrial carbon cycle to climate warming is positive, as models simulate a stronger warming stimulation of respiration than photosynthesis (1, 2). In contrast, results from experimental studies show that warming stimulates either net carbon release or uptake in terrestrial ecosystems, leading to positive (3–5) or negative (6, 7) climate-carbon feedbacks across different ecosystems (8), respectively. The mechanisms governing the observed variable feedbacks have not been well understood nor represented in models. Among many candidate mechanisms, soil water content (SWC) has been shown to ubiquitously regulate responses of the carbon cycle to warming in most experiments (5, 9–11). While a recent study in a boreal forest has showed that the effects of climate warming on plant photosynthesis flipped from positive during wet to negative during dry periods of the growing season (12), it is not clear whether such a flipping pattern of warming effects tipped by water conditions is generalizable to other carbon processes or even across ecosystem types.
It is generally known that ecosystem carbon processes, such as net ecosystem productivity (NEP) or soil respiration, elevate with SWC in dry environment, reach its maximum at the optimal condition, and then depress in water-logged environment (13, 14). Accordingly, we hypothesize that warming-induced decrease in SWC alleviates the depressing effects of water on NEP under wet conditions but aggravates drought effects under dry conditions. This indirect effect of warming through changes in SWC superimposes with direct warming effects on NEP to determine magnitudes and directions of carbon-climate feedback (Fig. 1).
Fig. 1. Conceptual diagram of warming effects on ecosystem C fluxes above and below the SWC optimum.
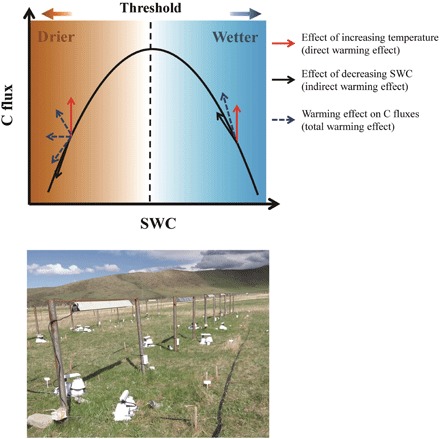
The red arrows represent the directly positive warming effect on ecosystem C fluxes. The black arrows represent the effect of warming-induced changes in SWC on C fluxes, which is the indirect effect of warming. Below the SWC optimum, the warming-induced decrease in SWC reduces C fluxes; thus, the black arrow points downward along the SWC-C flux response curve. Above the SWC threshold, warming-induced water loss increases C fluxes; thus, the indirect warming effect enhances C fluxes, and the black arrow points upward along the SWC-C flux response curve. The blue dashed arrows represent the final change direction of C fluxes under the combination of both direct and indirect effects of warming. The photograph depicts our field experimental plots at the study site. (Photo credit: Q.Q., Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences).
RESULTS AND DISCUSSION
To test the above hypothesis and to understand the underlying mechanisms, we conducted a field manipulation experiment in an alpine meadow of the Qinghai-Tibetan Plateau. The unique feature of this alpine meadow is a wide span of SWC from extremely wet to dry conditions and the high warming sensitivity (15, 16). Our experiment consisted of three temperature treatments, including ambient (C), +1.5°C (W1.5), and + 2.5°C (W2.5) soil temperatures (STs) at a depth of 10 cm. On average, across 3 years, warming significantly decreased SWC by 2.3 and 5.8%, respectively, with W1.5 and W2.5 treatments (fig. S1). Measured NEP, gross ecosystem productivity (GEP), and ecosystem respiration (ER) all showed diverse responses to warming among years (fig. S2) and among the seasons within the year (fig. S3). Across all the measurements, GEP, ER, and NEP followed hump-shaped response surfaces with ST and SWC (Fig. 2). The response surface resulted from a quadratic relationship of GEP, ER, and NEP with SWC and an exponential relationship with ST (Eq. 4 in the “Statistical analysis” section and fig. S4). A clear ridge along an SWC of 29.9 ± 2.4% (i.e., the optimum SWC) emerged from the hump-shaped response surfaces. GEP, ER, and NEP increased with SWC below its optimum but decreased above the optimum. The SWC optimum tended to shift toward larger values as temperature increased (Fig. 2).
Fig. 2. Response surfaces showing the relationships between ST and SWC versus GEP, ER, or NEP across plots and years.
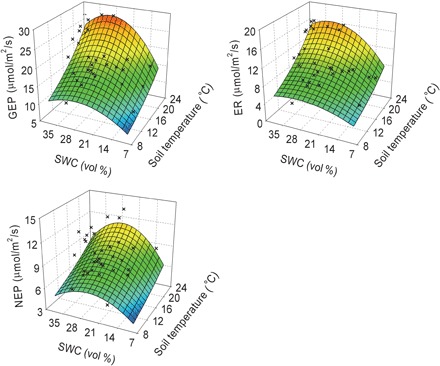
Observed values (black crosses) are the means of five replications of C fluxes, and modeled values (colored surfaces) are predictions from the models fitted with observations.
The hump-shaped response surfaces can help us to explain diverse warming effects on GEP, ER, and NEP with changing SWC (Fig. 3). When SWC was higher than its optimum (the ridge of the hump-shaped surface), warming-induced increase in ST (red arrows) and decrease in SWC (black arrows) both promoted GEP (blue dashed arrows represent their combined effects). When SWC was below the optimum, the combined positive impact of increasing temperature with negative impact of decreasing SWC under experiment warming resulted in either an increase or a decrease in GEP. As GEP response was always greater than that of ER in this study, the response pattern was similar for both GEP and NEP (Fig. 3). Warming alone consistently promotes plant growth and enhances GEP under adequate moisture availability (10, 17). Warming also accelerates microbial decomposition of soil organic C and thus increases ER (18–20). Under water deficits, the negative impacts of warming-induced droughts limit both carbon uptake and ER (5, 10, 21, 22). Since plant photosynthesis is more sensitive to drought than respiration (23, 24), GEP decreased more than ER, leading to a decrease in NEP and a positive carbon-climate feedback under low SWC.
Fig. 3. Response surfaces showing the relationships between ST and SWC versus ecosystem C fluxes (i.e., GEP, ER, and NEP) across plots and years.
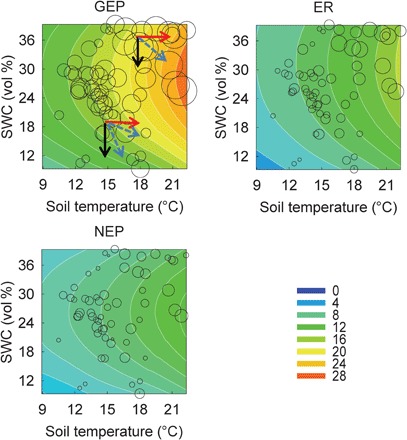
Observed values (bubbles) are the means of each measurement of GEP, ER, and NEP. Modeled values (colored response surfaces with contour lines) are predictions from the models fitted with observations. The red arrows represent the effects of increasing temperature, and the black arrows indicate those of warming-induced decrease in SWC. The blue dashed arrows represent the potential changes of ecosystem C fluxes in combination of the two driving forces of changing ST and SWC.
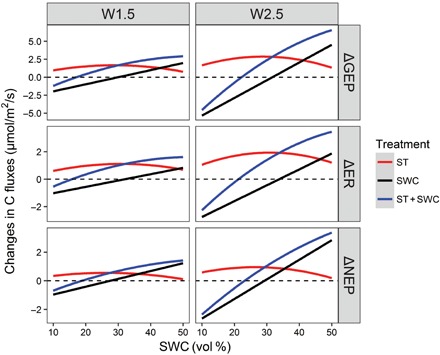
We used a nonlinear statistical model to discern the relative effects of warming-induced changes in temperature and SWC on GEP, ER, and NEP. Our analysis demonstrated that increasing ST under warming treatments alone always raised GEP, ER, and NEP across the full range of SWC (Fig. 4). However, warming-induced decreases in SWC stimulated GEP, ER, and NEP in its high range but depressed them in the low range (Fig. 4). Changes in these carbon fluxes formed linear scaling relationships with SWC. As a consequence, the warming effect was linearly scaled up or down with SWC, depending on which ranges the warming-induced decreases in SWC fell. Below the optimum, warming-induced decline in SWC reduced carbon fluxes to offset the stimulation caused by increasing ST, leading to either decreases in GEP, ER, and NEP under severe dry conditions or minor increases in these carbon fluxes under moderate dry conditions (Fig. 4). Above the optimum, warming-induced changes in SWC and ST both stimulated GEP, ER, and NEP. Their combination amplified each other to produce a much stronger stimulation than their individual effects (Fig. 4). While the patterns of water scaling were similar between the two levels of temperature treatments, the magnitudes of impacts were higher under the W2.5 treatment than those under the W1.5 treatment (Fig. 4).
Fig. 4. The simulated changes in GEP, ER, and NEP caused by warming-induced increasing ST (red line), warming-induced decrease in SWC (black line), and the combination effects of ST and SWC (ST + SWC, blue line) under two warming treatments (W1.5 and W2.5).
To test whether this water scaling pattern is generalizable across ecosystems, we conducted a global meta-analysis. We examined warming impacts on NEP in relation with ambient precipitation, due to fewer data points reported on warming-induced changes in SWC in the literatures from the study sites (table S1). However, precipitation is a good proxy for SWC at both site levels (figs. S5 and S6) and the global scale as shown in another synthesis study (25). In general, warming-induced changes in NEP were highly correlated with ambient precipitation (Fig. 5, P = 0.003) but not with the magnitude or duration of experimental warming (table S2) or mean annual temperature (table S2). This relationship holds even when experimental data only from the temperate zone were used and those from other climate zones (e.g., tundra ecosystems) were excluded. Overall, warming significantly reduced NEP under low precipitation but increased it under high precipitation.
Fig. 5. Global synthesis of experimental warming-induced relative changes in NEP with ambient precipitation.
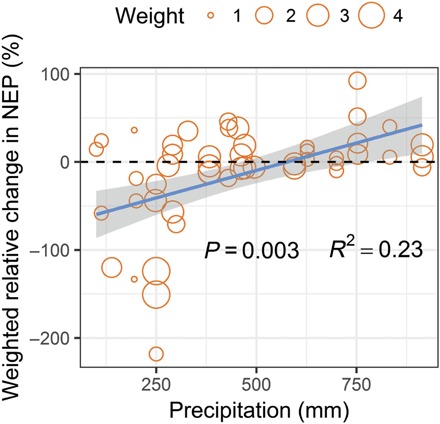
This water scaling can well explain conflicting results reported from previous case studies on warming-induced changes in ecosystem carbon fluxes (26, 27). Increases in plant growth, GEP, ER, or NEP under experimental warming were mostly detected in wet ecosystems, such as subtropical forests with a mean annual precipitation (MAP) of 1778 mm (28), temperate spruce forests with a MAP of 1480 mm (5), and alpine meadows with a MAP of 966 mm (29). In contrast, the warming-induced decreases in these ecosystem carbon fluxes were mainly reported in dry ecosystems, such as semiarid grasslands with a MAP of 375 and 241 mm (24, 30), high arctic tundra with a MAP of 197 mm (19), and high arctic polar semidesert with a MAP of 122 mm (10).
This study, to our knowledge, reconciles mechanisms revealed from site-level experiments with patterns of water scaling of warming effects at global scales. Our experiment in the Qinghai-Tibetan Plateau revealed a flipping pattern, as did from the boreal forest experiment (12), that warming effect was negative under dry conditions but positive under wet conditions. This flipping pattern is confirmed by our meta-analysis to be generalizable across experiments in different ecosystems. Our combined study suggests that water availability represents a strong, consistent mechanism underlying diverse, sometimes contradictory, responses of carbon processes to climate warming observed from different ecosystems.
Beyond revealing a generalizable pattern of water scaling, our study has also developed a continuous (i.e., quadratic) function (Eq. 4 in the
“Statistical analysis” section) to represent scaling of carbon fluxes with SWC. This function allowed us to identify thresholds of SWC between stimulating and inhibiting warming effects on carbon fluxes (Figs. 2 and 3). The threshold becomes higher under high (W2.5) than low (W1.5) temperature treatments. This continuous function also allowed us to attribute observed ecosystem responses to direct warming effects versus indirect warming effects through changes in SWC (Fig. 4). Direct warming effects at our alpine meadow site were always positive along SWC gradients for all the GEP, NEP, and ER, whereas indirect warming effects through changes in SWC were positive under wet conditions but negative under dry conditions. This water scaling function has the potential to improve model prediction of carbon cycle feedback to climate change. Models have used a variety of response functions, such as linear (31), Gaussian (32), exponential (33), and reverse exponential (34), to represent carbon cycle responses to SWC. These functions are apparently not sufficient to represent a full spectrum of C cycle responses to water availability. For example, the linear response function is likely to work well when one ecosystem is prevailingly under either dry or wet condition. In comparison, this study reveals that a continuous function is needed to represent a full spectrum of hydrological regulation of the C cycle and its feedback to climate warming to improve model prediction of C cycle responses to climate warming (35).
Our findings shed light on future climate-carbon feedback at least in a couple of ways. Future warmer conditions will lead to variable feedbacks of ecosystems to climate warming, depending on moisture conditions. Ecosystems in very dry regions most likely decrease carbon uptake under warming conditions and thus cause a positive feedback to climate warming. In contrast, ecosystems in wet regions possibly generate a negative feedback. Moreover, warmer climate will results in chronically lowering soil moisture. Lowering soil moisture will stimulate warming effects in wet regions but exert a strong braking effect on, or even reverse, the potential benefit of climate warming on ecosystem carbon uptake in dry regions. Thus, this and other studies suggest precipitation thresholds to regulate NEP variation at regional and continental scales (36).
In summary, our and many other field warming experiments provide compelling evidence on water regulation of ecosystem feedback to climate warming at both temporal and spatial scales. The revealed water scaling represents a generalizable pattern for understanding ecosystem-climate feedback from local to global scales. A continuous function of water scaling is needed to describe observed shifts of carbon-climate feedback from being negative to positive along the full spectrum of water availability at our experimental site and likely other sites. This water scaling pattern is generally supported by the global meta-analysis that warming stimulates net ecosystem carbon release (i.e., positive carbon cycle feedbacks to climate warming) in low-precipitation regions but enhances carbon uptake (i.e., negative feedbacks) in high-precipitation regions. This water scaling pattern enriches mechanistic understanding of temperature-moisture interactions in affecting ecosystem carbon cycling and its feedback to climate change and has the potential to improve model prediction.
MATERIALS AND METHODS
Study area for the warming experiment
The experimental study was conducted in an alpine meadow of the Eastern Qinghai-Tibetan Plateau (32°48′N, 102°58′E), which locates in Hongyuan County, Sichuan of China, at an altitude of approximately 3500 m. Over the past 60 years, the MAP is 753 mm, with approximately 80% occurring during May to September, and the mean annual temperature is 1.1°C, with January as the coldest month (−10.3°C) and July as the hottest month (10.9°C). The soil at the study site is classified as Cryumbrept following the U.S. Soil Taxonomy (37, 38). Plant species in this alpine meadow are dominated by Deschampsia caespitosa (Linn.) Beauv., Koeleria cristata (Linn.) Pers., Gentiana sino-ornata Balf. f., Potentilla anserina L., and Anemone rivularis Buch.-Ham.
Experimental design
We used random block design with three warming treatments and five replications for each treatment in this study. Three 3 m by 2 m plots were laid out in each of the five blocks and randomly assigned to the three treatments of control (C), low-level warming (W1.5), and high-level warming (W2.5). The warmed plots were continuously heated, proceeding in June 2014, via 165 cm by 15 cm infrared radiators (MSR-2420, Kalglo Electronics Inc., Bethlehem, Pennsylvania, USA), which were suspended in the center of the plot, at 1.5 m above ground level. The heaters for the W1.5 treatments were set at an output power of approximately 1000 W, with the expectation of a 1.5°C increase in ST at 10-cm depth, while the heaters for the W2.5 treatments were set at an output power of approximately 2000 W, with the expectation of a 3°C increase in ST. In each control plot, we suspended a dummy heater whose appearance is identical to the infrared radiator at the same height to simulate the shading effect. The adjacent plots were 3 m apart.
Measurement of ecosystem CO2 fluxes
We measured NEP and ER twice per month during the growing season on clear days from June to September in 2014 to 2016. In May 2014, we installed a 0.5 m by 0.5 m square aluminum frame into the soil at the depth of 3 cm in each plot, with a distance of at least 30 cm from the perimeter of the plot, to seal the canopy chamber (0.5 m by 0.5 m by 0.5 m, polymethyl methacrylate) to the soil surface and provided a plane interface between them. Care was taken to minimize soil disturbance during the installation. NEP and ER were measured using an infrared gas analyzer (LI-6400XT, LI-COR Environmental, Lincoln, Nebraska, USA), which was attached to the transparent canopy chamber. During measurements, two small fans were installed diagonally inside the chamber and fanned continuously to mix the atmosphere. Consecutive recordings of CO2 concentrations were obtained once every 10 s in 80 s. NEP was calculated by the slope between recording time and concentrations [see the detailed method in (11)]. Right after the NEP measurements, the chamber was lifted up to exchange air with the outside. Then, we covered an opaque cloth on the chamber and repeated the measurement to obtain ER. GEP was calculated as the difference between NEP and ER.
ST (°C) was measured at a 10-cm depth using a thermocouple probe concurrently with the ecosystem CO2 fluxes measurements. The 10-cm SWC (%V) was measured using a time domain reflectometry instrumentation (TDR 100, Spectrum Technologies Inc., Chicago, USA). All measurements were performed at the same time with C fluxes measurement.
Meta-analysis
To analyze the effect of precipitation on warming-induced changes in NEP at global scale, we synthesized various warming experiments involved in terrestrial ecosystems. First, we used “Web of Science” and “Google Scholar” to search peer-reviewed literatures that investigated NEP response under experimental warming during 1900–2019. Then, we screened the papers for analysis based on the criteria as follows. (i) Field studies must include a control and warming treatment under the same condition between them. (ii) The variable of NEP is shown by its mean and sample size. (iii) Experimental method needs to be explicitly described as well, such as warming magnitude, experimental duration, and warming method. Data shown in figures were extracted with the Engauge Digitizer (Free Software Foundation Inc., Boston, MA, USA). If multiple years’ data were reported in the same experiment, we only selected the latest measurement to assure statistical independence between observations. Overall, we established a global dataset composed of 34 independent experiments (including this study), with warming magnitude ranging from 0.2° to 3°C and experimental duration from 1 to 9 years. Since this study examines the response of NEE, we only included the direct measurement of NEE that was conducted by chambers in herbaceous ecosystem, including tall-grass prairie, temperate steppe, peatland, fens, alpine meadow, and tundra, with precipitation range from 100 to 914 mm and temperature from −14.6° to 16.3°C (table S1).
Warming effect was calculated as (NEPtreatment − NEPcontrol)/NEPcontrol. These effects were weighted by their sample size, Ntreatment × Ncontrol/(Ntreatment + Ncontrol), where Ntreatment and Ncontrol represent the sample size of NEP in warming and control treatment, respectively. We analyzed the relationship between warming-induced changes in NEP and ambient precipitation with a linear mixed-effect model using the lme4 package (39) in R 3.4.1 for Windows. Precipitation was considered as fixed effect, and studies were considered as random effect to interpret possible autocorrelation among observations in each experiment. Moreover, we also used a linear mixed-effect model to analyze the interactions of precipitation, temperature, warming magnitude, and experimental duration on NEP change (table S2). Similarly, precipitation, temperature, warming magnitude, and experimental duration were considered as fixed effect with a random effect of studies.
We used three ways to justify that MAP is an adequate proxy for soil moisture. First, by substituting SWC with precipitation, we verified that the water-scaling patterns still existed firmly in this study. The warming effects on GEP, ER, and NEP varied from negative to positive when monthly precipitation changed from low to high in our site-level experiment (fig. S5). Second, we used data from a precipitation gradient experiment, which is adjacent to our warming experiment (less than 10 m) (40). We found that precipitation was an adequate proxy for SWC at our study site (fig. S6). Third, at the global scale, previous studies indicate that MAP is a good proxy for soil moisture across large space or various experiments (25).
Statistical analyses
Repeated-measures analysis of variance (ANOVA) was used to explore the effects of warming, year, and their interactions on GEP, ER, and NEP (table S3) and those of measurement time, warming, and their interactions on C fluxes over the growing season for each year (table S4). On the basis of the relationship of GEP, ER, or NEP with ST (fig. S4) (6), we used an exponential function to describe their relationships.
| (1) |
A quadratic function was used to analyze the relationships between these C fluxes and SWC.
| (2) |
For the relationships between C fluxes and the interaction of ST and SWC, both linear and nonlinear models were tried and compared. The linear model assumed that GEP, ER, and NEP were linearly dependent on the SWC.
| (3) |
The nonlinear model assumed that ecosystem C fluxes depended on the product of two terms: (i) an increasing exponential function of ST and (ii) a threshold quadratic function of SWC.
| (4) |
where Fc represents GEP, ER, or NEP, ST and SWC represent ST and SWC, respectively, and γs are the fitted model coefficients.
We performed model selection based on the Akaike information criterion (AIC). Because the nonlinear model (Eq. 4) consistently gave lower AIC (table S5), we selected the nonlinear threshold model, as shown in Eq. 4. The quadratic coefficients (γ1) for SWC were significantly different from zero for GEP, ER, and NEP (table S6), indicating bell-shaped response patterns. We validated the nonlinear model (Eq. 4) by comparing the observed and modeled values. The comparison showed that the model fitted the observations well, with a 49 to 76% agreement for different C fluxes (fig. S7).
We were aware that seasonality might bias the relationships between SWC and GEP, ER, or NEP. To evaluate the potential impacts of this bias, we used peak growing season (July and August) data and performed the same analyses. The results showed the same threshold response of C fluxes to SWC (fig. S8), which further justified the nonlinear threshold responses of ecosystem C fluxes to SWC.
Then, we used the model (Eq. 4) to distinguish the impacts of warming-induced changes in ST, SWC, and their interactions on GEP, ER, and NEP (Fig. 4). On average, ST was around 15°C in the control across the 3 years under the current study. Thus, we set 15°C as the baseline ambient ST [ST(15)] and modeled the effects of warming-induced changes in ST and SWC on GEP, ER, and NEP at different SWC levels with 5% intervals (10, 15, 20, 25, 30, 35, 40, 45, and 50%). At each SWC [SWC(S)] level, the impacts of warming-induced changes in ST were quantified by
| (5) |
where Fc represents the function of model (4) and ΔST represents warming-induced increases in ST.
The influence of warming-induced changes in SWC on GEP, ER, and NEP was calculated as
| (6) |
where ΔSWC represents the warming-induced decrease in SWC.
The combined effects of warming-induced changes in ST and SWC were calculated as
| (7) |
where ΔST and ΔSWC were, on average, the differences in ST and SWC of each plot between warming and control treatment across 3 years.
Supplementary Material
Acknowledgments
We thank the staff of Institute of Qinghai-Tibetan Plateau in Southwest University for Nationalities. Funding: This study was financially supported by the Ministry of Science and Technology of China (2016YFC0501803), The Second Tibetan Plateau Scientific Expedition and Research (STEP) program (2019QZKK0302), the National Natural Science Foundation of China (31625006 and 31470528), and the “Thousand Youth Talents Plan.” K.Z. was supported by a Faculty Research Grant awarded by the Committee on Research from the University of California, Santa Cruz. Author contributions: S.N. and Q.Q. conceived the ideas, designed the study, and led the writing of the manuscript. Q.Q., D.T., F.Z., and Q.Z. collected the data. Q.Q. and D.T. analyzed the data. All authors contributed to the drafts and revision and gave the final approval for publication. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/8/eaav1131/DC1
Table S1. Site information in global meta-analysis.
Table S2. Effects of mean annual precipitation (MAP), mean annual temperature (MAT), experimental warming magnitude, duration, and their interactions on relative changes in NEP across global herbaceous ecosystems with a linear mixed-effect model.
Table S3. Repeated-measures ANOVA results (F values) on the effects of warming (W), year (Y), and their interactions on GEP, ER, NEP, ST (Tsoil), and moisture (Msoil).
Table S4. Repeated-measures ANOVA results (F values) on the effects of warming (W), measured time (T), and their interactions on GEP, ER, NEP, ST (Tsoil), and moisture (Msoil) in 2014–2016.
Table S5. Comparison of the nonthreshold and threshold models based on the AIC for GEP, ER, and NEP.
Table S6. Coefficients of threshold model (means and 95% confidence intervals) (Eq. 4).
Fig. S1. Seasonal dynamics and means of ST and SWC at 10-cm depth under three warming treatments in 2014 to 2016.
Fig. S2. Seasonal means of GEP, ER, and NEP under different warming treatments in 2014 to 2016.
Fig. S3. Warming-induced changes in GEP, ER, and NEP within the year.
Fig. S4. Relationships between ST and GEP, ER, or NEP across seasons and plots.
Fig. S5. Relationships between warming-induced response ratio of ecosystem carbon fluxes with monthly precipitation.
Fig. S6. The relationship between response ratio of monthly mean SWC and monthly precipitation in a precipitation gradient experiment at our study site from 2015 to 2016.
Fig. S7. Relationships between the modeled and observed values of carbon fluxes with 1:1 line.
Fig. S8. Relationships between SWC and ecosystem C fluxes within peak growing seasons (July and August).
REFERENCES AND NOTES
- 1.Friedlingstein P., Cox P., Betts R., Bopp L., Von Bloh W., Brovkin V., Cadule P., Doney S., Eby M., Fung I., Bala G., John J., Jones C., Joos F., Kato T., Kawamiya M., Knorr W., Lindsay K., Matthews H. D., Raddatz T., Rayner P., Reick C., Roeckner E., Schnitzler K. G., Schnur R., Strassmann K., Weaver A. J., Yoshikawa C., Zeng N., Climate-carbon cycle feedback analysis: Results from the (CMIP)-M-4 model intercomparison. J. Clim. 19, 3337–3353 (2006). [Google Scholar]
- 2.Friedlingstein P., Meinshausen M., Arora V. K., Jones C. D., Anav A., Liddicoat S. K., Knutti R., Uncertainties in CMIP5 climate projections due to carbon cycle feedbacks. J. Clim. 27, 511–526 (2014). [Google Scholar]
- 3.Crowther T. W., Todd-Brown K. E. O., Rowe C. W., Wieder W. R., Carey J. C., Machmuller M. B., Snoek B. L., Fang S., Zhou G., Allison S. D., Blair J. M., Bridgham S. D., Burton A. J., Carrillo Y., Reich P. B., Clark J. S., Classen A. T., Dijkstra F. A., Elberling B., Emmett B. A., Estiarte M., Frey S. D., Guo J., Harte J., Jiang L., Johnson B. R., Kroel-Dulay G., Larsen K. S., Laudon H., Lavallee J. M., Luo Y., Lupascu M., Ma L. N., Marhan S., Michelsen A., Mohan J., Niu S., Pendall E., Penuelas J., Pfeifer-Meister L., Poll C., Reinsch S., Reynolds L. L., Schmidt I. K., Sistla S., Sokol N. W., Templer P. H., Treseder K. K., Welker J. M., Bradford M. A., Quantifying global soil carbon losses in response to warming. Nature 540, 104–108 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Liu W., Zhang Z., Wan S., Predominant role of water in regulating soil and microbial respiration and their responses to climate change in a semiarid grassland. Glob. Chang. Biol. 15, 184–195 (2009). [Google Scholar]
- 5.Schindlbacher A., Wunderlich S., Borken W., Kitzler B., Zechmeister-Boltenstern S., Jandl R., Soil respiration under climate change: Prolonged summer drought offsets soil warming effects. Glob. Chang. Biol. 18, 2270–2279 (2012). [Google Scholar]
- 6.Zhang Z., Zhang R., Cescatti A., Wohlfahrt G., Buchmann N., Zhu J., Chen G., Moyano F., Pumpanen J., Hirano T., Takagi K., Merbold L., Effect of climate warming on the annual terrestrial net ecosystem CO2 exchange globally in the boreal and temperate regions. Sci. Rep. 7, 3108 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Orangeville L., Duchesne L., Houle D., Kneeshaw D., Côte B., Pederson N., Northeastern North America as a potential refugium for boreal forests in a warming climate. Science 352, 1452–1455 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Luo Y., Terrestrial carbon-cycle feedback to climate warming. Annu. Rev. Ecol. Evol. Syst. 38, 683–712 (2007). [Google Scholar]
- 9.Wan S., Norby R. J., Ledford J., Weltzin J. F., Responses of soil respiration to elevated CO2, air warming, and changing soil water availability in a model old-field grassland. Glob. Chang. Biol. 13, 2411–2424 (2007). [Google Scholar]
- 10.Sharp E. D., Sullivan P. F., Steltzer H., Csank A. Z., Welker J. M., Complex carbon cycle responses to multi-level warming and supplemental summer rain in the high Arctic. Glob. Chang. Biol. 19, 1780–1792 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Niu S., Wu M., Han Y., Xia J., Li L., Wan S., Water-mediated responses of ecosystem carbon fluxes to climatic change in a temperate steppe. New Phytol. 177, 209–219 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Reich P. B., Sendall K. M., Stefanski A., Rich R. L., Hobbie S. E., Montgomery R. A., Effects of climate warming on photosynthesis in boreal tree species depend on soil moisture. Nature 562, 263–267 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Davidson E. A., Belk E., Boone R. D., Soil water content and temperature as independent or confounded factors controlling soil respiration in a temperate mixed hardwood forest. Glob. Chang. Biol. 4, 217–227 (1998). [Google Scholar]
- 14.Taylor P. G., Cleveland C. C., Wieder W. R., Sullivan B. W., Doughty C. E., Dobrowski S. Z., Townsend A. R., Temperature and rainfall interact to control carbon cycling in tropical forests. Ecol. Lett. 20, 779–788 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Yang Y., Fang J., Tang Y., Ji C., Zheng C., He J., Zhu B., Storage, patterns and controls of soil organic carbon in the Tibetan grasslands. Glob. Chang. Biol. 14, 1592–1599 (2008). [Google Scholar]
- 16.Peng F., Xu M., You Q., Zhou X., Wang T., Xue X., Different responses of soil respiration and its components to experimental warming with contrasting soil water content. Arct. Antarct. Alp. Res. 47, 359–368 (2015). [Google Scholar]
- 17.Xue K., Yuan M. M., Shi Z. J., Qin Y. J., Deng Y., Cheng L., Wu L. Y., He Z., Van Nostrand J. D., Bracho R., Natali S., Schuur E. A. G., Luo C., Konstantinidis K. T., Wang Q., Cole J. R., Tiedje J. M., Luo Y., Zhou J., Tundra soil carbon is vulnerable to rapid microbial decomposition under climate warming. Nat. Clim. Chang. 6, 595–600 (2016). [Google Scholar]
- 18.Melillo J. M., Frey S. D., DeAngelis K. M., Werner W. J., Bernard M. J., Bowles F. P., Pold G., Knorr M. A., Grandy A. S., Long-term pattern and magnitude of soil carbon feedback to the climate system in a warming world. Science 358, 101–104 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Glanville H. C., Hill P. W., Maccarone L. D., Golyshin P. N., Murphy D. V., Jones D. L., Temperature and water controls on vegetation emergence, microbial dynamics, and soil carbon and nitrogen fluxes in a high Arctic tundra ecosystem. Funct. Ecol. 26, 1366–1380 (2012). [Google Scholar]
- 20.Treseder K. K., Marusenko Y., Romero-Olivares A. L., Maltz M. R., Experimental warming alters potential function of the fungal community in boreal forest. Glob. Chang. Biol. 22, 3395–3404 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Albert K. R., Ro-Poulsen H., Mikkelsen T. N., Michelsen A., Van der Linden L., Beier C., Effects of elevated CO2, warming and drought episodes on plant carbon uptake in a temperate heath ecosystem are controlled by soil water status. Plant Cell Environ. 34, 1207–1222 (2011). [DOI] [PubMed] [Google Scholar]
- 22.Kim J.-S., Kug J.-S., Jeong S.-J., Huntzinger D. N., Michalak A. M., Schwalm C. R., Wei Y. X., Schaefer K., Reduced North American terrestrial primary productivity linked to anomalous Arctic warming. Nat. Geosci. 10, 572–576 (2017). [Google Scholar]
- 23.Shi Z., Thomey M. L., Mowll W., Litvak M., Brunsell N. A., Collins S. L., Pockman W. T., Smith M. D., Knapp A. K., Luo Y., Differential effects of extreme drought on production and respiration: Synthesis and modeling analysis. Biogeosciences 11, 621–633 (2014). [Google Scholar]
- 24.Li G., Han H., Du Y., Hui D., Xia J., Niu S., Li X., Wan S., Effects of warming and increased precipitation on net ecosystem productivity: A long-term manipulative experiment in a semiarid grassland. Agric. For. Meteorol. 232, 359–366 (2017). [Google Scholar]
- 25.Liu L., Wang X., Lajeunesse M. J., Miao G., Piao S., Wan S., Wu Y., Wang Z., Yang S., Li P., Deng M., A cross-biome synthesis of soil respiration and its determinants under simulated precipitation changes. Glob. Chang. Biol. 22, 1394–1405 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Mekonnen Z. A., Grant R. F., Schwalm C., Contrasting changes in gross primary productivity of different regions of North America as affected by warming in recent decades. Agric. For. Meteorol. 218–219, 50–64 (2016). [Google Scholar]
- 27.Wang X., Liu L., Piao S., Janssens I. A., Tang J., Liu W., Chi Y., Wang J., Xu S., Soil respiration under climate warming: Differential response of heterotrophic and autotrophic respiration. Glob. Chang. Biol. 20, 3229–3237 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Wu C., Liang N., Sha L., Xu X., Zhang Y., Lu H., Song L., Song Q., Xie Y., Heterotrophic respiration does not acclimate to continuous warming in a subtropical forest. Sci. Rep. 6, 21561 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winkler D. E., Chapin K. J., Kueppers L. M., Soil moisture mediates alpine life form and community productivity responses to warming. Ecology 97, 1553–1563 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Wertin T. M., Belnap J., Reed S. C., Experimental warming in a dryland community reduced plant photosynthesis and soil CO2 efflux although the relationship between the fluxes remained unchanged. Funct. Ecol. 31, 297–305 (2017). [Google Scholar]
- 31.Leirós M. C., Trasar-Cepeda C., Seoane S., Gil-Sotres F., Dependence of mineralization of soil organic matter on temperature and moisture. Soil Biol. Biochem. 31, 327–335 (1999). [Google Scholar]
- 32.Vicca S., Bahn M., Estiarte M., Van Loon E. E., Vargas R., Alberti G., Ambus P., Arain M. A., Beier C., Bentley L. P., Borken W., Buchmann N., Collins S. L., de Dato G., Dukes J. S., Escolar C., Fay P., Guidolotti G., Hanson P. J., Kahmen A., Kröel-Dulay G., Ladreiter-Knauss T., Larsen K. S., Lellei-Kovacs E., Lebrija-Trejos E., Maestre F. T., Marhan S., Marshall M., Meir P., Miao Y., Muhr J., Niklaus P. A., Ogaya R., Peñuelas J., Poll C., Rustad L. E., Savage K., Schindlbacher A., Schmidt I. K., Smith A. R., Sotta E. D., Suseela V., Tietema A., van Gestel N., van Straaten O., Wan S., Weber U., Janssens I. A., Can current moisture responses predict soil CO2 efflux under altered precipitation regimes? A synthesis of manipulation experiments. Biogeosciences 11, 2991–3013 (2014). [Google Scholar]
- 33.Rodrigo A., Recous S., Neel C., Mary B., Modelling temperature and moisture effects on C–N transformations in soils: Comparison of nine models. Ecol. Model. 102, 325–339 (1997). [Google Scholar]
- 34.Zhou X., Wan S., Luo Y., Source components and interannual variability of soil CO2 efflux under experimental warming and clipping in a grassland ecosystem. Glob. Chang. Biol. 13, 761–775 (2007). [Google Scholar]
- 35.Suseela V., Conant R. T., Wallenstein M. D., Dukes J. S., Effects of soil moisture on the temperature sensitivity of heterotrophic respiration vary seasonally in an old-field climate change experiment. Glob. Chang. Biol. 18, 336–348 (2012). [Google Scholar]
- 36.Liu Z., Ballantyne A. P., Poulter B., Anderegg W. R. L., Li W., Bastos A., Ciais P., Precipitation thresholds regulate net carbon exchange at the continental scale. Nat. Commun. 9, 3596 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li G., Sun S., Plant clipping may cause overestimation of soil respiration in a Tibetan alpine meadow, southwest China. Ecol. Res. 26, 497–504 (2011). [Google Scholar]
- 38.Shi C., Silva L. C. R., Zhang H., Zheng Q., Xiao B., Wu N., Sun G., Climate warming alters nitrogen dynamics and total non-structural carbohydrate accumulations of perennial herbs of distinctive functional groups during the plant senescence in autumn in an alpine meadow of the Tibetan Plateau, China. Agric. For. Meteorol. 200, 21–29 (2015). [Google Scholar]
- 39.Bates D., Mächler M., Bolker B. M., Walker S., Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015). [Google Scholar]
- 40.Zhang F., Quan Q., Ma F., Tian D., Zhou Q., Niu S., Differential responses of ecosystem carbon flux components to experimental precipitation gradient in an alpine meadow. Funct. Ecol. 33, 889–900 (2019). [Google Scholar]
- 41.Johnson C. P., Pypker T. G., Hribljan J. A., Chimner R. A., Open top chambers and infrared lamps: A comparison of heating efficacy and CO2/CH4 dynamics in a northern michigan peatland. Ecosystems 16, 736–748 (2013). [Google Scholar]
- 42.Peng F., You Q., Xu M., Guo J., Wang T., Xue X., Effects of warming and clipping on ecosystem carbon fluxes across two hydrologically contrasting years in an alpine meadow of the Qinghai-Tibet Plateau. PLOS ONE 9, e109319 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ganjurjav H., Gao Q., Zhang W., Liang Y., Li Y., Cao X., Wan Y., Li Y., Danjiu L., Effects of warming on CO2 fluxes in an alpine meadow ecosystem on the central Qinghai-Tibetan Plateau. PLOS ONE 10, e0132044 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang L., Guo R., Zhu T., Niu X., Guo J., Sun W., Water- and plant-mediated responses of ecosystem carbon fluxes to warming and nitrogen addition on the Songnen grassland in northeast China. PLOS ONE 7, e45205 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pearson M., Penttilä T., Harjunpää L., Laiho R., Laine J., Sarjala T., Silvan K., Silvan N., Effects of temperature rise and water-table-level drawdown on greenhouse gas fluxes of boreal sedge fens. Boreal Environ. Res. 20, 489–505 (2015). [Google Scholar]
- 46.Kim M. K., Henry H. A. L., Net ecosystem CO2 exchange and plant biomass responses to warming and N addition in a grass-dominated system during two years of net CO2 efflux. Plant Soil 371, 409–421 (2013). [Google Scholar]
- 47.Oberbauer S. F., Tweedie C. E., Welker J. M., Fahnestock J. T., Henry G. H. R., Webber P. J., Hollister R. D., Walker M. D., Kuchy A., Elmore E., Starr G., Tundra CO2 fluxes in response to experimental warming across latitudinal and moisture gradients. Ecol. Monogr. 77, 221–238 (2007). [Google Scholar]
- 48.Niu S., Sherry R. A., Zhou X., Luo Y., Ecosystem carbon fluxes in response to warming and clipping in a tallgrass prairie. Ecosystems 16, 948–961 (2013). [Google Scholar]
- 49.Oechel W. C., Vourlitis G. L., Hastings S. J., Ault R. P. Jr., Bryant P., The effects of water table manipulation and elevated temperature on the net CO2 flux of wet sedge tundra ecosystems. Glob. Chang. Biol. 4, 77–90 (1998). [Google Scholar]
- 50.Grogan P., Chapin F. S. III, Initial effects of experimental warming on above- and belowground components of net ecosystem CO2 exchange in arctic tundra. Oecologia 125, 512–520 (2000). [DOI] [PubMed] [Google Scholar]
- 51.Niu S., Xing X., Zhang Z., Xia J., Zhou X., Song B., Li L., Wan S., Water-use efficiency in response to climate change: From leaf to ecosystem in a temperate steppe. Glob. Chang. Biol. 17, 1073–1082 (2011). [Google Scholar]
- 52.Chen J., Luo Y., Xia J., Wilcox K., Cao J., Zhou X., Jiang L., Niu S., Estera K. Y., Huang R. J., Wu F., Hu T. F., Liang J. Y., Shi Z., Guo J. F., Wang R.-W., Warming effects on ecosystem carbon fluxes are modulated by plant functional types. Ecosystems 20, 515–526 (2017). [Google Scholar]
- 53.Wu Q., Han G.-D., Wang Z.-w., Pan Z.-l., Liu F., Wang R.-z., Zhang R.-y., Qin J., Li J.-w., Effects of warming and N addition on ecosystem carbon exchange in a desert steppe. Chinese J. Ecol. 35, 1427–1434 (2016). [Google Scholar]
- 54.Hobbie S. E., Chapin F. S. III, Response of tundra plant biomass, aboveground production, nitrogen, and CO2 flux to experimental warming. Ecology 79, 1526–1544 (1998). [Google Scholar]
- 55.Welker J. M., Fahnestock J. T., Henry G. H. R., O’Dea K. W., Chimner R. A., CO2 exchange in three Canadian High Arctic ecosystems: Response to long-term experimental warming. Glob. Chang. Biol. 10, 1981–1995 (2004). [Google Scholar]
- 56.Xia J., Niu S., Wan S., Response of ecosystem carbon exchange to warming and nitrogen addition during two hydrologically contrasting growing seasons in a temperate steppe. Glob. Chang. Biol. 15, 1544–1556 (2009). [Google Scholar]
- 57.Xu X., Shi Z., Chen X., Lin Y., Niu S., Jiang L., Luo R., Luo Y., Unchanged carbon balance driven by equivalent responses of production and respiration to climate change in a mixed-grass prairie. Glob. Chang. Biol. 22, 1857–1866 (2016). [DOI] [PubMed] [Google Scholar]
- 58.Peng F., Xue X., Xu M., You Q., Jian G., Ma S., Warming-induced shift towards forbs and grasses and its relation to the carbon sequestration in an alpine meadow. Environ. Res. Lett. 12, 044010 (2017). [Google Scholar]
- 59.Munir T. M., Perkins M., Kaing E., Strack M., Carbon dioxide flux and net primary production of a boreal treed bog: Responses to warming and water-table-lowering simulations of climate change. Biogeosciences 12, 1091–1111 (2015). [Google Scholar]
- 60.Yan W. C., Sun G., Zhang C. B., He J., Zhang N. N., Impacts of experimental warming and moderate grazing on ecosystem carbon exchange and its compositions in an alpine meadow on the eastern Qinghai-Tibetan Chinese. J. Appl. Environ. Biol. 24, 132–139 (2018). [Google Scholar]
- 61.Huemmrich K. F., Kinoshita G., Gamon J. A., Houston S., Kwon H., Oechel W. C., Tundra carbon balance under varying temperature and moisture regimes. J. Geophys. Res. 115, G00I02 (2010). [Google Scholar]
- 62.Liu J., Chen K. L., Zhang L. L., Response of ecosystem carbon exchange to warming during the growing season of alpine lake wetland. Qinghai Prataculture 27, 2–8 (2018). [Google Scholar]
- 63.Ganjurjav H., Hu G., Wan Y., Li Y., Danjiu L., Gao Q., Different responses of ecosystem carbon exchange to warming in three types of alpine grassland on the central Qinghai-Tibetan Plateau. Ecol. Evol. 8, 1507–1520 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Z. F. Chen, Master’s thesis, Inner Mongolia Agricultural University (2012). [Google Scholar]
- 65.J. Song, Master’s thesis, Henan University (2015). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/8/eaav1131/DC1
Table S1. Site information in global meta-analysis.
Table S2. Effects of mean annual precipitation (MAP), mean annual temperature (MAT), experimental warming magnitude, duration, and their interactions on relative changes in NEP across global herbaceous ecosystems with a linear mixed-effect model.
Table S3. Repeated-measures ANOVA results (F values) on the effects of warming (W), year (Y), and their interactions on GEP, ER, NEP, ST (Tsoil), and moisture (Msoil).
Table S4. Repeated-measures ANOVA results (F values) on the effects of warming (W), measured time (T), and their interactions on GEP, ER, NEP, ST (Tsoil), and moisture (Msoil) in 2014–2016.
Table S5. Comparison of the nonthreshold and threshold models based on the AIC for GEP, ER, and NEP.
Table S6. Coefficients of threshold model (means and 95% confidence intervals) (Eq. 4).
Fig. S1. Seasonal dynamics and means of ST and SWC at 10-cm depth under three warming treatments in 2014 to 2016.
Fig. S2. Seasonal means of GEP, ER, and NEP under different warming treatments in 2014 to 2016.
Fig. S3. Warming-induced changes in GEP, ER, and NEP within the year.
Fig. S4. Relationships between ST and GEP, ER, or NEP across seasons and plots.
Fig. S5. Relationships between warming-induced response ratio of ecosystem carbon fluxes with monthly precipitation.
Fig. S6. The relationship between response ratio of monthly mean SWC and monthly precipitation in a precipitation gradient experiment at our study site from 2015 to 2016.
Fig. S7. Relationships between the modeled and observed values of carbon fluxes with 1:1 line.
Fig. S8. Relationships between SWC and ecosystem C fluxes within peak growing seasons (July and August).


