Figure 2. Model of lysozyme-mediated σV activation.
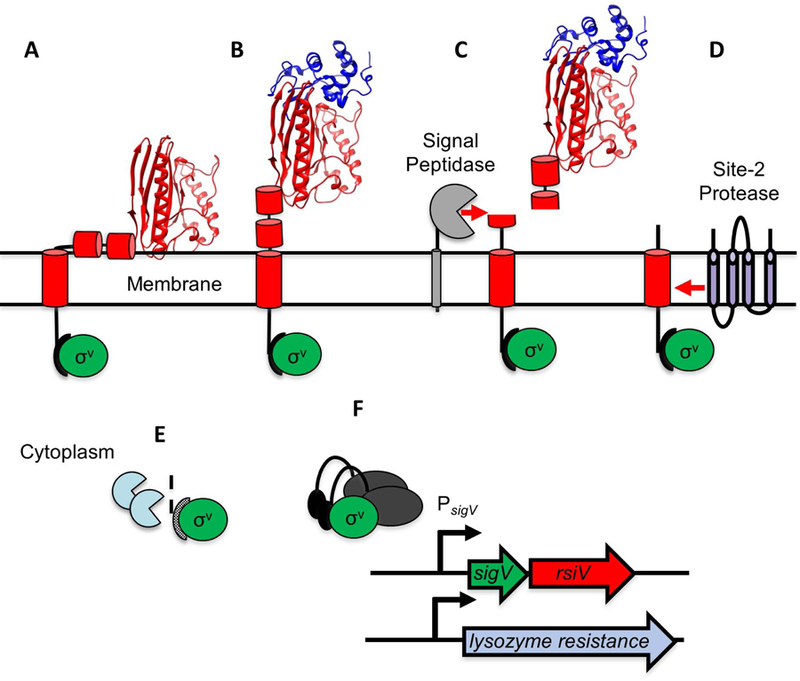
A. In the absence of lysozyme, RsiV (red) binds σV (green). Two amphipathic helices of RsiV (short red cylinders) are embedded in the membrane. B. Lysozyme (blue ribbon diagram) binds the C-terminal extracytoplasmic portion of RsiV (red ribbon diagram) and alters the structure of RsiV such that the amphipathic helices are no longer membrane-embedded. C. Signal peptidase (grey) can recognize and cleave RsiV at site-1 within the first amphipathic helix. D. The site-2 protease (RasP/Eep in purple) cleaves the site-1 cleavage product within the transmembrane domain. E. Cytoplasmic proteases (light blue) further degrade the RsiV site-2 cleavage product. F. σV (green), no longer bound to RsiV, is free to interact with RNA polymerase and σV promoters.
