Figure 3. Structural comparison of two ECF30 anti-σ factors.
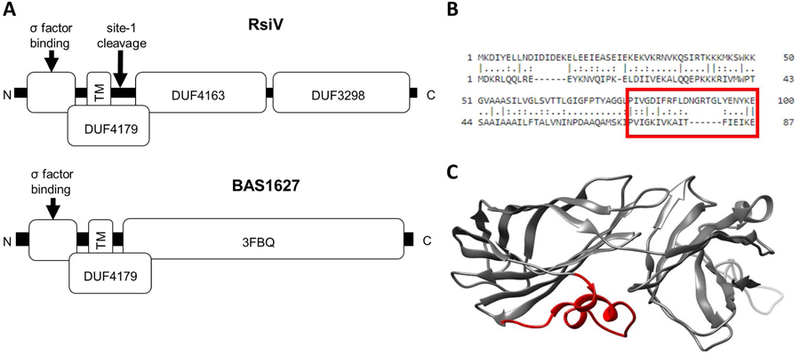
A. Domain structure of two ECF30 family anti-σ factors. RsiV (top) consists of 3 domains of unknown function. DUF4163 and DUF3298 comprise the lysozyme binding domain of RsiV. DUF4179 includes the transmembrane domain, amphipathic helices and site-1 cleavage site. The bottom portion shows BAS1627 an anti-σ factor from B. anthracis. The 3FBQ portion represents the solved X-ray crystal structure of BAS1627 and contains the c-terminal portion of the DUF4179. B. Alignment of RsiV and BAS1627. The region boxed in red are the amphipathic helices of RsiV. C. The structure of BAS1627 (3FBQ). The regions in red are the amphipathic helices that correspond the boxed region in B.
