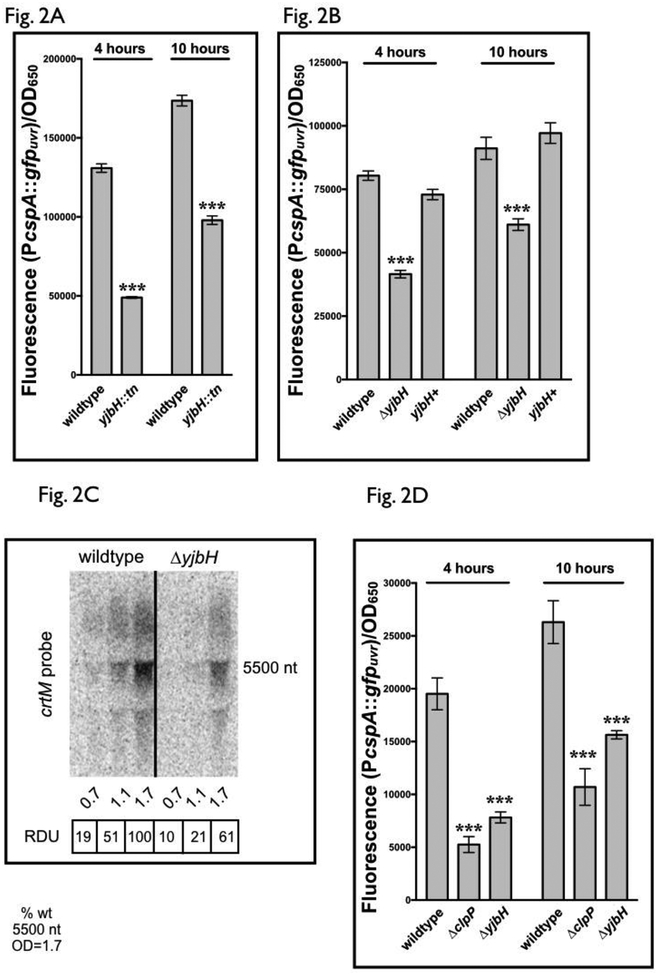
Figure 2: yjbH regulates cspA promoter activity in S. aureus.
(A) cspA promoter activity in S. aureus JE2 wildtype and a strain disrupted for yjbH by the mariner transposon. GFP fluorescence generated from the cspA promoter was measured at 4 and 10 hours for S. aureus JE2 wildtype and NE896 (yjbH transposon mutant), carrying the plasmid pALC8135 with cspA promoter driving gfp. Cell densities were simultaneously measured at OD650 and fluorescence normalized to OD650. The value for each strain represents the mean of two biological replicates that were read in triplicate, and the experiments were repeated three times. A representative experiment is displayed.
(B) cspA promoter activity in S. aureus SH1000 wildtype, de-novo constructed ΔyjbH strains and yjbH mutant with chromosomal replacement. Fluorescence of S. aureus SH1000 wildtype, ΔyjbH and yjbH+, its chromosomally complemented strain, carrying the plasmid pALC8135 was measured at 4 and 10 hours of growth in TSB. Cell densities were simultaneously measured at OD650 and fluorescence normalized by it. The value for each strain represents the mean read in triplicate and the experiments were repeated three times. A representative experiment is displayed. The yjbH+ strain indicates the yjbH complement strain ALC8121.
(C) A representative Northern blot of the crtOPQMN transcript in SH1000 wildtype and ΔyjbH strains. RNA obtained from SH1000 wildtype and ΔyjbH cells at various optical densities (15 μg of cellular RNA per lane) was resolved on a denaturing agarose gel, blotted to Hybond XL membrane and hybridized with a 300-bp 32P-radiolabeled crtM DNA probe. The relative densitometric units (RDU) of each ~5.5k nt band were calculated relative to wildtype at OD650=1.7. The experiment was repeated at least two times with consistent results.
(D) cspA promoter activity in S. aureus SH1000 wildtype and strains deleted for ΔclpP and ΔyjbH. Fluorescence of S. aureus SH1000 wildtype, ΔclpP and ΔyjbH carrying the plasmid pALC8135 was measured at 4 and 10 hours of growth in TSB. Cell densities were simultaneously measured at OD650 and fluorescence normalized by it. The value for each strain represents the mean of two biological replicates that were read in triplicate, and the experiments were repeated three times. A representative experiment is displayed.
The asterisks in Fig. 2A, 2B and 2D indicate statistical significance between wildtype and mutant strains, determined using Student t-test (***, P <0.0005).