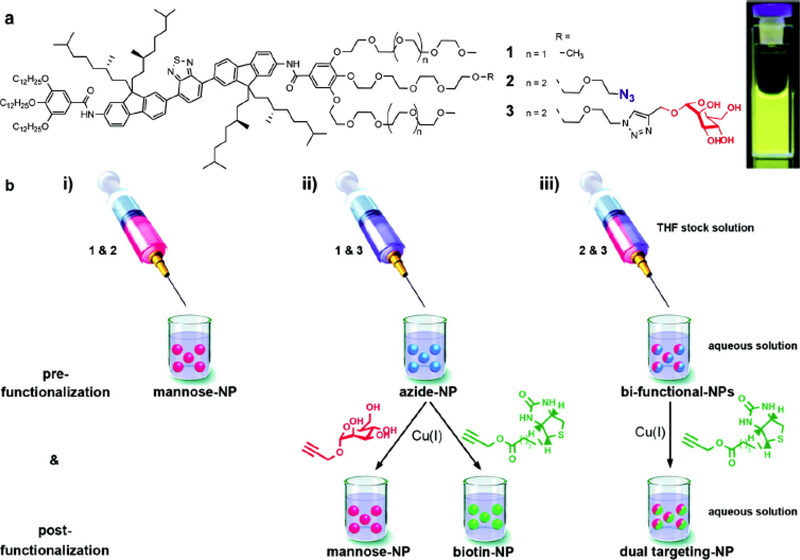
Fig. (3).
(a) Chemical structures of amphiphiles and a photograph of a 0.3 μM nanoparticle solution under UV light (360 nm). (b)(i) Generation of nanoparticles prefunctionalized with mannose using mixtures of 1 and 2. (ii) Generation of nanoparticles prefunctionalized with azides using mixtures of 1 and 3 and their subsequent postfunctionalization via copper catalyzed azide-alkyne cycloaddition with alkyne derivatives of either mannose or biotin. (iii) Generation of bifunctional nanoparticles containing azides and mannose using mixtures of 2 and 3 and their subsequent postfunctionalization via copper catalyzed azide-alkyne cycloaddition with alkyne derived biotin yielding dual targeting mannose and biotin labeled nanoparticles (reproduced with permission from Petkau et al. [32] Copyright © American Chemical Society, 2011).