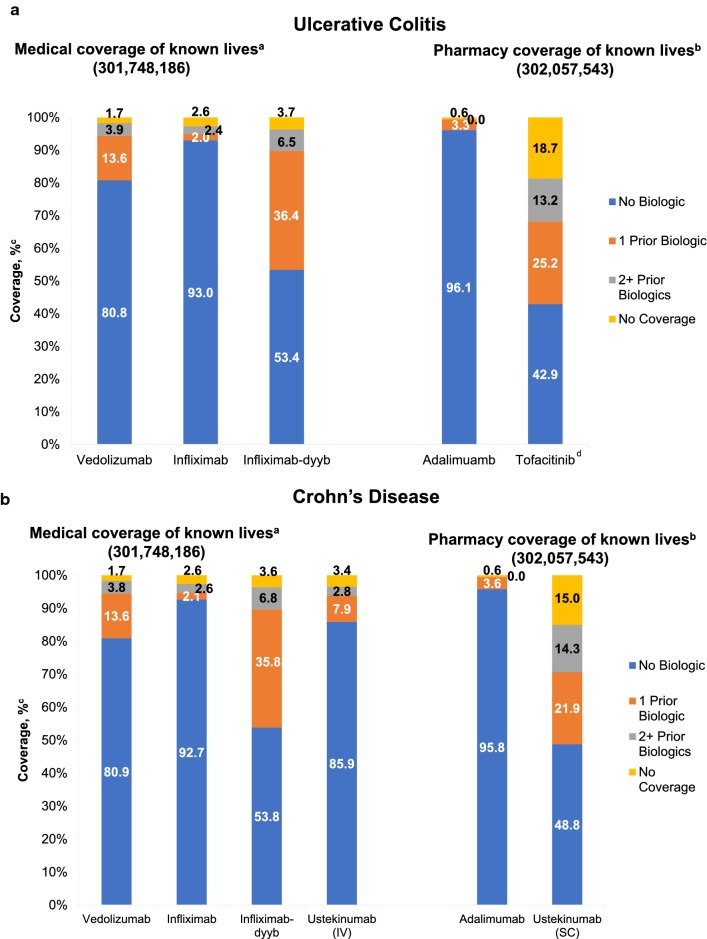
Fig. 2.
Drug access under medical coverage for ulcerative colitis and Crohn’s disease. aApproximately 7% and 8% of lives had unknown coverage under medical benefit in UC and CD, respectively. bApproximately 16% and 7% of lives had unknown coverage under pharmacy benefit in UC and CD, respectively. cThe calculated percentage of covered lives excluded all unknown lives. dTofacitinib has a large percentage of unknown lives (~ 25%), primarily because it was only approved for UC in May 2018. Therefore, this may result in overinflection of the “No Biologic” coverage. CD, Crohn’s disease; MMIT, Managed Markets Insight and Technology; UC, ulcerative colitis
Source: MMIT, December 18, 2018