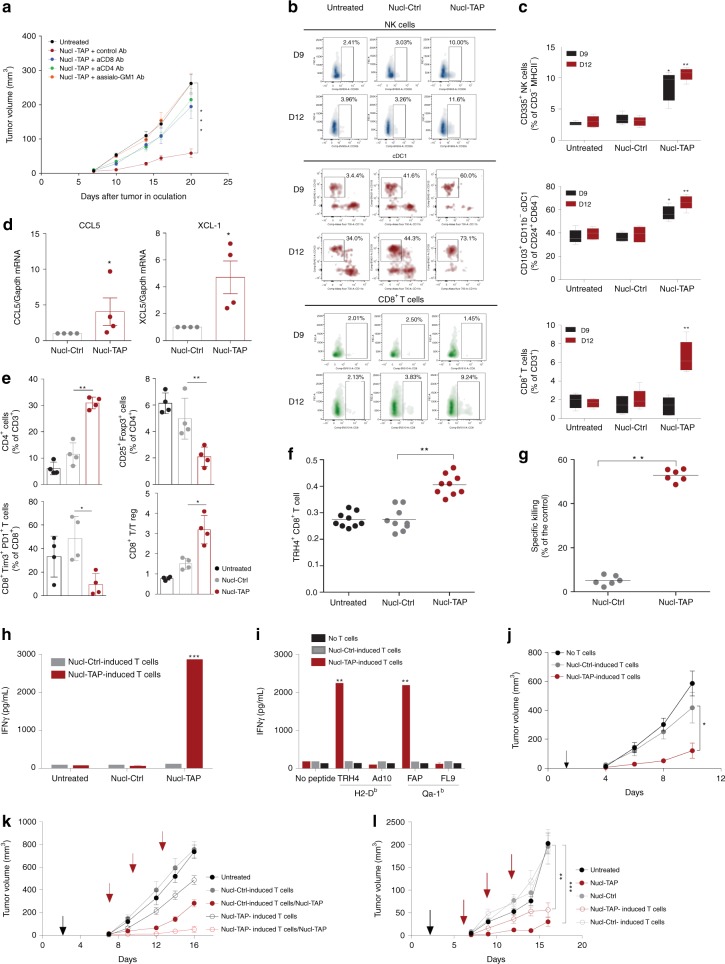
Fig. 4.
Immune responses elicited in mice treated with Nucl-TAP. a CD4+ T cell, CD8+ T cell, and NK cells dependence of tumor inhibition. 4T1 tumor-bearing mice treated with Nucl-TAP, and anti-CD4, anti-CD8, or anti-asialo-GM1 antibodies (6 mice/group). Data represent means and SEM (n = 2). b and c Accumulation of cDC1, NK and CD8+ T cells at day 9 and day 12 in 4T1 tumors after the first and second dose of Nucl-TAP siRNA administration, respectively. b Individual mice represented as density plots. c Distribution within a group of four mice represented as box plot analysis (see gating strategy in Supplementary Fig. 4a, b). d Secretion of XCL-1 and CCL5 in day 9 4T1 tumors. Data represent means and SEM. e Tumor-infiltrating immune subsets. 4T1 tumors were resected from mice 2 days after second dose of Nucl-TAP and immune cells subsets were analyzed by flow cytometry. Data represent mean and SEM (4 mice/group) (n = 2). f and g Treatment of RMA tumor-bearing mice with Nucl-TAP elicits TRH4-specific CD8+ T cell responses. f Staining with TRH4-H-2Db tetramer on tumor-infiltrating lymphocytes 2 days after second dose administration (9 mice/group) (n = 2). g In vivo killing of TRH4 peptide pulsed splenocytes as described in the “Methods” section. Each circle represents an individual mouse, and means per group are shown. h and i CD8+ T cells isolated from MC38 tumor-bearing mice treated with Nucl-TAP recognize in vitro RMA tumor cells incubated with Nucl-TAP h or DC2.4 cells pulsed with the TAP downregulation induced Kb-restricted TRH4 peptide and Qa-1b restricted FAP peptide i. IFNγ production after 20 h stimulation was measured by ELISA. Means and SEM of triplicate wells are represented (n = 2). j Adoptive transfer of TAP-specific CD8+ T cells from MC38 bearing mice treated with Nucl-TAP inhibits the growth of the TAP-deficient RMA-S tumors. Arrows: T cells infusion (7 mice/group). Data represent means and SEM (n = 1). k and l TAP downregulation mediated induction of non-TAP deficient specific CD8+ T antitumor immunity. Adoptive transfer of CD8+ T cells from RMA k or 67NR l bearing mice treated with Nucl-TAP inhibits the growth of these tumors in the absence of Nucl-TAP treatment. Black arrow, T cells infusion: red arrows, Nucl-siRNAs treatment (6–9 mice/group). Data represent means and SEM (n = 1). For k, statistical analysis at day 16: Nucl-Ctrl-induced T cells versus Nucl-TAP-induced T cells, P = 0.0060; Nucl-Ctrl-induced T cells versus Nucl-Ctrl-induced T cells/Nucl-TAP, p < 0.0001; Nucl-Ctrl-induced T cells versus Nucl-TAP-induced T cells/Nucl-TAP, P < 0.0001; Nucl-TAP-induced T cells versus Nucl-Ctrl-induced T cells/Nucl-TAP, n.s; Nucl-Ctrl-induced T cells/Nucl-TAP versus Nucl-TAP-induced T cells/Nucl-TAP, n.s; Nucl-TAP-induced T cells versus Nucl-TAP-induced T cells/Nucl-TAP; P < 0.0001. a, c, e, h–l Statistical analyses using one-way ANOVA test and Tuckey posttest. d Statistical analyses using Mann–Whitney test. f and g Statistical analyses using Kruskal–Wallis and Dunn posttest. c, h, and l Comparisons between Nucl-Ctrl versus Nucl-TAP-treated/induced cells. a, c–j, l differences are indicated in graphs: ***P < 0.005, **P < 0.01, *P < 0.05