Figure 1.
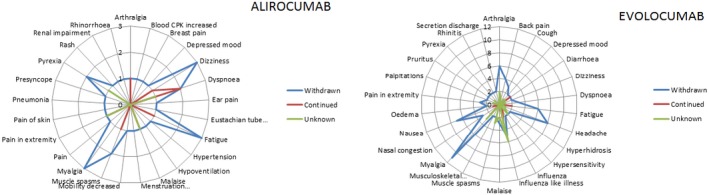
Drug action taken for alirocumab and evolocumab in Lareb database. Drug action taken per reported adverse event (AE) by patients who discontinued alirocumab (left) and evolocumab (right) in the Lareb database compared to patients who continued or for whom drug action was unknown. Axis shows number of patients who reported a specific AE. AEs with a frequency of n = 1 have been excluded for evolocumab to allow for better overview and visualization of the most common AEs.
