Figure 3.
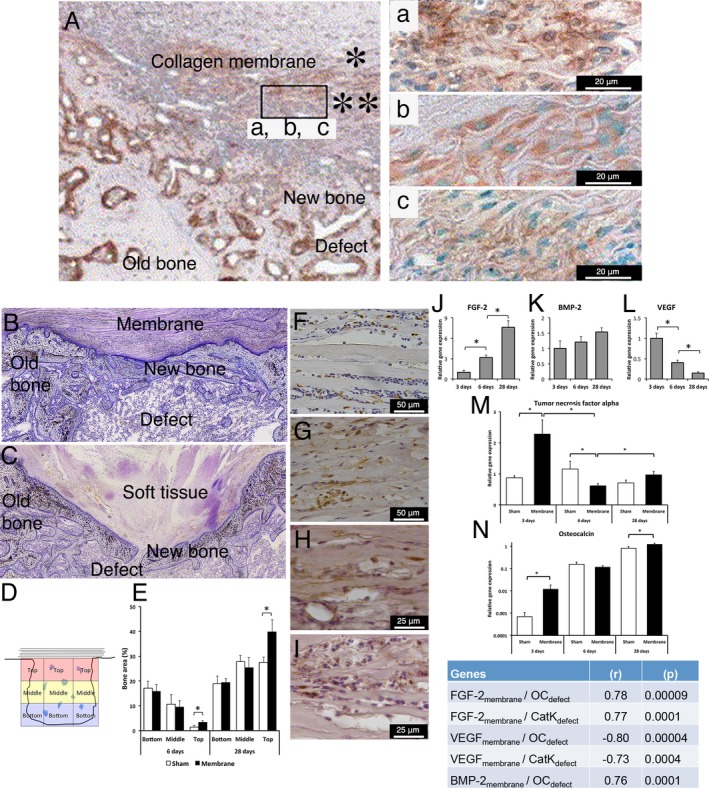
Cellular, molecular and structural events during guided bone regeneration (GBR) by collagen‐based membranes on surgically created bone defects in a rat model. (A) Immunohistochemical findings in decalcified and paraffin‐embedded sections showing GBR using a porcine type I/III collagen membrane consisting of a “compact” top part (*) and a “porous” bottom part (**) placed on a surgically created maxillary defect in the rat model after 2 weeks of healing. In (A), abundant newly formed bone (NB) is observed filling the defect, and an abundant cell infiltrate is observed in the porous part of the membrane facing the defect. The insert shows that the cells infiltrating the porous part of the porcine type I/III collagen membrane are positively stained for the bone proteins (a) alkaline phosphatase (ALP), (b) osteopontin (OPN) and (c) osteocalcin (OC), suggesting the active participation of the membrane‐associated cells in the bone regeneration process. (B and C) Histological images of undecalcified, resin‐embedded and toluidine blue‐stained sections showing that the application of a collagen membrane (derived from porcine small intestine extracellular matrix (ECM)) to a rat femur bone defect (B) results in structural restitution of the underlying defect with newly formed bone compared to the lesser restitution in the untreated defect (C). In the untreated sham defect (C), soft tissue invasion and poor restitution of the defect are evident. The histomorphometric analysis of the different regions of the defect (D and E) demonstrates a higher proportion of newly regenerated bone in the defect treated with the ECM collagen membrane than in the untreated sham defect, specifically in the top region of the defect directly underneath the membrane. The immunohistochemical analyses show that during GBR (exemplified here at 3 days), different cell types, including CD68‐positive macrophages (F) and periostin‐positive osteoprogenitor cells (G), are recruited and hosted within the ECM collagen membrane above the defect. Moreover, the immunohistochemical analysis reveals positive protein reactivity for the pro‐osteogenic, bone‐promoting growth factors FGF‐2 (H) and BMP‐2 (I) inside the membrane. The molecular analysis (qPCR) confirms the progressively increasing expression of the bone‐promoting growth factors FGF‐2 (J) and BMP‐2 (K) in conjunction with a temporal downregulation of the vascularization growth factor VEGF (L) in the membrane‐associated cells. The corresponding molecular qPCR analysis of the underlying defect reveals that the application of the ECM collagen membrane modulates the molecular activities of different healing processes, exemplified here by the pro‐inflammatory cytokine TNF‐α (M) and the bone formation gene OC (N), providing molecular evidence for membrane‐promoted bone healing and regeneration in the underlying defect. The significant correlations between the gene expression in the membrane and the gene expression in the underlying defect (insert table) demonstrate that the molecular activities in the two compartments are linked during the course of GBR. The upper panel of the figure (A and a, b c) is adapted and reprinted from Biomaterials, 26 (31), Taguchi Y, Amizuka N, Nakadate M, Ohnishi H, Fujii N, Oda K, Nomura S, Maeda T., A histological evaluation for guided bone regeneration induced by a collagenous membrane., 6158‐66, copyright (2005), with permission from Elsevier [via the Copyright Clearance Center]. The lower panel of the figure is adapted and reprinted from the Eur J Oral Sci, 125 (5), Elgali I, Omar O, Dahlin C, Thomsen P, Guided bone regeneration: materials and biological mechanisms revisited., 315–337, copyright (2017), published by John Wiley & Sons Ltd. under a Creative Commons license (CC‐BY‐NC‐ND): https://creativecommons.org/licenses/by-nc-nd/4.0/
