Figure 3.
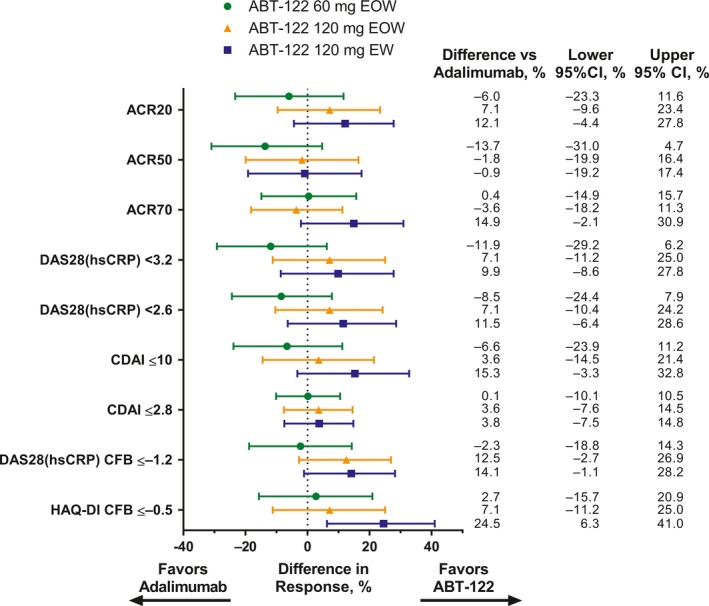
Differences in efficacy end points at 12 weeks between the ABT‐122 dosing groups compared with the adalimumab group. The end points include the American College of Rheumatology response criteria for an improvement of at least 20% (ACR20), 50% (ACR50), and 70% (ACR20), 2 score cutoffs for the Disease Activity Score in 28 joints based on high‐sensitivity C‐reactive protein level (DAS28‐hsCRP), 2 score cutoffs for the Clinical Disease Activity Index (CDAI), and change from baseline (CFB) of ≤−1.2 in the DAS28‐hsCRP score or ≤−0.5 on the disability index (DI) of the Health Assessment Questionnaire (HAQ). Bars show the difference in response with 95% confidence interval (95% CI). EOW = every other week; EW = every week.
