Abstract
A large body of the literature has demonstrated that the polysialic acid (polySia) modification of the neural cell adhesion molecule (NCAM) is a key regulator of cellular interactions during brain development, maintenance and plasticity. To properly fulfill these functions, polySia concentration has to be carefully controlled. This is done by the regulation of the expression of the two polySia-synthesizing enzymes ST8SiaII and ST8SiaIV. From this point of view we and others have demonstrated that downregulation of ST8SiaIV during oligodendrocyte differentiation is a prerequisite for efficient myelin formation and maintenance. Here, we addressed the question whether the prevention of polySia downregulation in neurons affects brain and particularly myelin development and functioning. For this purpose, we developed transgenic (tg) mouse lines overexpressing the polysialyltransferase ST8SiaIV in neurons. tg expression of ST8SiaIV prevented the postnatal downregulation of polySia, and most of the polySias in the forebrain and brain stem of adult tg mice were associated with NCAM-140 and NCAM-180 isoforms. Structural examination of the brain revealed no overt abnormalities of axons and myelin. In addition, ultrastructural and western blot analyses indicated normal myelin development. However, behavioral studies revealed reduced rearing activity, a measure for exploratory behavior, while parameters of motor activity were not affected in tg mice. Taken together, these results suggest that a persisting presence of polySia in neurons has no major effect on brain structure, myelination and myelin maintenance, but causes mild behavioral changes.
Keywords: brain development, exploratory behavior, motor coordination, myelin structure, polysialic acid, polysialyltransferase ST8SiaIV, transgenic mice
Introduction
Neural plasticity mediated through the neural cell adhesion molecule (NCAM) is facilitated by post-translational modifications, the most important and prevalent of which is glycosylation with polysialic acid (polySia) (Finne et al. 1983; Gascon et al. 2007). PolySia is a linear homopolymer of α-2,8-linked sialic acid with a degree of polymerization ranging from 8 to >90 units and found almost exclusively associated with NCAM (reviewed by Hildebrandt and Dityatev, 2015). The carbohydrate is added post-translationally by two Golgi-associated polysialyltransferases (ST8SiaII and ST8SiaIV), which are expressed in a spatiotemporally regulated manner in the central nervous system (CNS) (Mühlenhoff et al. 1998; Hildebrandt et al. 1998; Angata and Fukuda, 2003). PolySia expression reduces not only cell adhesion mediated by NCAM but also interactions of other adhesion molecules (Johnson et al. 2005) and modulates functions such as cell migration and axonal growth in the developing brain (Zhang et al. 1992; Decker et al. 2000).
In mammals, polySia expression is widespread during embryonic and early postnatal brain development, and almost all neurons are positive for polySia at some stage of differentiation (Probstmeier et al. 1994; Ong et al. 1998; Bonfanti, 2006). More specifically, a study on the development of mesencephalic-dopaminergic system in mice has shown that mRNA expression of both polysialyltransferases in the ventral mid brain as well as in the whole brain significantly increases after embryonic day (E) 10.5, reaches a plateau between E13.5 and E14.5 and is then maintained until birth (Schiff et al. 2009). During postnatal mouse brain development, the level of both enzymes is significantly down-regulated. However, ST8SiaIV displays a moderate decline, contrasted by a sharp reduction of ST8SiaII mRNA between postnatal days 5 and 11, followed by a significant decline of polySia (Oltmann-Norden et al. 2008; Hildebrandt and Dityatev, 2015). In adulthood, polySia levels are negligible in most brain areas, with the exception of sites of neural plasticity and neurogenesis such as the subventricular zone and the dentate gyrus of the hippocampus (Seki and Arai, 1993; Gage, 2000; Bernier et al. 2002; Luzzati et al. 2003).
The mammalian brain develops in a predictable sequence that includes myelination, which is essential for motor, sensory and higher-order cognitive function (Bercury and Macklin, 2015). The process of myelination is tightly regulated by several intrinsic signaling molecules as well as polySia, which is expressed on the surface of both neurons and oligodendrocytes (Miller 2002; Mitew et al. 2013; Schnaar et al. 2014; Szewczyk et al. 2017). Removal of polySia as well as loss of ST8SiaIV accelerates differentiation of oligodendrocyte precursors in vitro and remyelination under pathological conditions in vivo (Decker et al. 2002; Koutsoudaki et al. 2010; Werneburg et al. 2017). Moreover, the analysis of mice expressing ST8SiaIV under the control of the proteolipid protein promoter demonstrated that the postnatal down-regulation of polySia by cells of the oligodendrocyte lineage is essential for efficient myelination (Fewou et al. 2007). On the other hand, myelination also coincides with a decrease of axonal polySia (Jakovcevski et al. 2007), and re-expression of polySia by demyelinated axons in multiple sclerosis is discussed as a mechanism that inhibits remyelination (Charles et al. 2002). However, it has not yet been addressed experimentally in vivo if downregulation of specifically the axonal polySia is relevant for myelination.
PolySia is also essential for neuroblast migration, differentiation and axon fasciculation (Hildebrandt et al. 2007; Gascon et al. 2007; Rutishauser, 2008; Hildebrandt and Dityatev, 2015). The outstanding role of polySia in controlling brain development first became apparent by enzymatic removal of polySia (see Rutishauser & Landmesser, 1996 for an early review) and later on was corroborated by genetic ablation of polysialyltransferases (Angata et al. 2004; Weinhold et al. 2005; Angata et al. 2007). ST8SiaII and ST8SiaIV double-deficient mice that are completely devoid of polySia synthesis displayed postnatal growth retardation and premature death, a high incidence of progressive hydrocephalus, smaller olfactory bulbs (obs) as well as defects of some, but not all, major brain fiber tracts, including anterior commissure (ac), internal capsule (ic), mamillothalamic and corticospinal tract (cst), and hippocampal (hp) mossy fiber projections (Weinhold et al. 2005). Interestingly, only some of these features recapitulate the phenotype of NCAM-deficient mice, whereas most are caused by the impaired balance between polySia and NCAM expression (Hildebrandt et al. 2009). In addition to playing a key role in brain development, numerous studies demonstrate a crucial role of polySia in behavioral plasticity. For example, impaired exploratory behavior is a hallmark of NCAM-deficient mice (Cremer et al. 1994), whereas increased rearing activity in the open-field test indicates a higher exploratory drive in the ST8SiaII-deficient mouse model (Angata et al. 2004).
Here we generated transgenic (tg) mice overexpressing the polysialyltransferase ST8SiaIV in neurons and studied its effect on polySia and NCAM expression, on morphological features of brain development with a particular focus on myelination, as well as on locomotors performance, motor coordination and exploratory drive. Although polySia in the brain of tg mice was significantly up-regulated at adult age, no defect was observed in brain morphology, myelination and myelin structure. This is consistent with the absence of deficits in motor coordination (rotarod test) and locomotor activity (open field). However, tg mice showed a significant deficit in vertical activity (rearing) indicating a reduction of exploratory drive. These results suggest that a persisting presence of polySia in neurons has no major effect on brain structure and myelination, but causes mild behavioral changes.
Results
ST8SiaIV tg mice express polySia in the gray matter
In order to prevent the postnatal down-regulation of polySia in neurons, tg mice were generated in which the Thy1.2 promoter, which has been demonstrated to drive the transgene expression in cerebral cortical and hp pyramidal neurons, ventrobasal thalamic neurons, subthalamic neurons and spinal motor neurons, but not in granule and purkinje cells of the cerebellum (Wang et al. 2011), controlled the expression of polysialyltransferase ST8SiaIV in neurons (Figure 1A). Several tg founder mice were identified by Southern blot analysis (data not shown), and two tg lines (tg4717 and tg4720) were established and maintained on an inbred C57BL/6 genetic background. Expression of the transgene was examined by northern blotting using a ST8SiaIV-specific cDNA probe (Figure 1B). Result showed a significant up-regulation of the transgene expression when comparing wild-type (wt) and tg littermates. In contrast, the endogenous ST8SiaIV-expression was down-regulated as the development of the CNS proceeded. In situ hybridizations with a digoxigenin-labeled ST8SiaIV cRNA probe indicated the transgene expression in neurons of the cortex (Figure 1C-D), hippocampus (Figure 1E-F) and thalamus (Figure 1G-H) in adult tg mice. Moreover, transgene expression was also detectable in the retina ganglion cell layer (Figure 1I-J) and in the spinal cord (Figure 1K-L). In contrast, transgene expression was not detectable in the Purkinje and granule cells of the cerebellum. The absence of transgene expression in the cerebellum is in line with previous reports on other tg lines using the same Thy1.2 promoter construct (see van der Putten et al. 2000, Figure 1). In contrast, only few neurons in the adult wt mice were found to be ST8SiaIV-positive (Figure 1D, F, H) as expected.
Fig. 1.
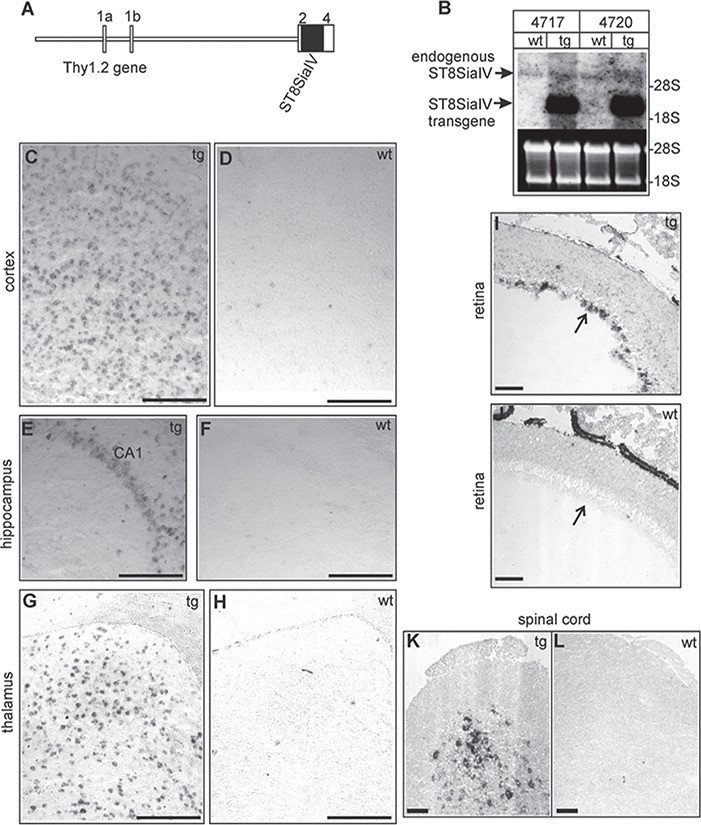
Generation of ST8SiaIV-tg mice. (A) Schematic representation of the tg construct. The murine ST8SiaIV cDNA was subcloned into the unique XhoI site of the Thy1.2 gene cassette (van der Putten et al., 2000) and is indicated by a solid box. Exons of the Thy1.2 gene are numbered 1a to 4 and are indicated by open boxes. (B) Northern blot analysis of tg mice of lines tg4717, tg4720 and wt littermates. Total RNA from tg and wt mouse brains (10 μg/lane) were separated by agarose gel electrophoresis, transferred to nylon membranes and hybridized to the ST8SiaIVspecific probe (entire coding sequence, black bar in A). Equal loading was controlled by ethidium bromide (EtBr) staining.(C–L) In situ hybridization of adult tg (linetg4717; tg) and wt was done on parasagittal brain sections, which were hybridized to ST8SiaIV specific antisense digoxigenin-labeled cRNA probes. Transgene expression was observed in the cortex (C), the hippocampal CA1region (E) and the laterodorsal thalamic nucleus (G). In wt mice only few cells gave hybridization signals (D, F, H). Hybridization of retina cryo-sections (age: P12) revealed transgene expression in the retina ganglion cell layer (arrow) in tg (I) but not in wt (J) mice. Transgene expression was also observed in the spinal cord of tg mice (K). ST8SiaIV expression was not detectable in wt spinal cord cross sections (L). (Control hybridizations with ST8SiaIV sense probes gave no specific hybridization signals (data not shown). Scale bars, 100 μm.
To further demonstrate that integration of the polysialyltransferase in the mouse genome resulted in the upregulation of polySia expression in neurons, we performed an immunoblot analysis of proteins from total brain homogenates. Results revealed an increase in the amount of polySia in tg mice as compared to wt littermates. The difference was less pronounced in young (11- to 21-day-old) mice (Figure 2A), which was expected because of the high polySia level at this developmental stage in the wt brain. Probing the membranes with anti-NCAM antibodies showed a significant decrease in the NCAM-140 and NCAM-180 bands due to the molecular mass shift of polysialylated NCAM proteins (Figure 2A). In adult mice (120 days), polySia concentration was strongly reduced in wt mice, whereas in tg mice polySia remained at a higher concentration (Figure 2A). NCAM-140 and NCAM-180 were hardly detectable in adult tg brains, indicating that these molecules were highly polysialylated, and the high amount of polySia carried by NCAM-140 and NCAM-180 in tg mice prevented the NCAM antibody from recognizing the NCAM protein. Because of the mass heterogeneity of polySia, NCAM from tg mice migrated as an unfocused smear with a high molecular mass (Figure 2A). PolySia was also increased in the optic nerve of adult (4 months old) tg mice (Figure 2B), which is in line with an elevated polySia level in axonal membranes. In order to confirm that the apparent decrease in NCAM proteins was only due to polysialylation and not down-regulation of NCAM expression, samples from different brain regions (fb, forebrain; cb, cerebellum; sb, brain stem) were treated with endoneuraminidase NF (endoN), which specifically cleaves polySia (Mühlenhoff et al., 2003). As shown in Figure 2C, after endoN treatment NCAM levels appeared to be similar in wt and tg mice in all brain regions examined. These western blots also showed that there was no significant increase of polySia in the cerebellum (Figure 2C), in accordance with the absence of transgene expression. In addition, the same western blot confirmed that increase in polySia affected only the NCAM isoforms 140 and 180 since they appear unfocussed in the brain samples (fb and sb) not treated with endoN, in contrast to NCAM-120 (which is the major NCAM isoform in myelin/oligodendrocytes), which appeared clearly focused in all conditions (Figure 2C).
Fig. 2.
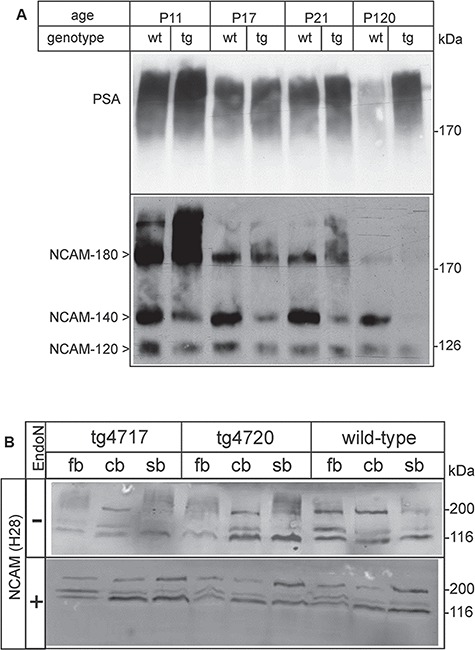
Expression of polySia in the brain of tg and wt mice. (A) Brains were isolated from wt and tg littermates (tline tg4717) at11, 17, 21 and 120 days of age. Total membrane fractions were prepared, separated (50 μg of protein/lane) in 7% SDS polyacrylamide gels and transferred onto nitrocellulose membranes. Membranes were probed with monoclonal antibodies 735 and H28, to detect polySia and NCAM protein, respectively. (B) Optic nerves of 4-months-old tg mice and wt littermates were analyzed by Western blotting (15 μg/lane) with antibodies against PSA (735, upper panel) and NCAM (H28, lower panel). The major signal corresponds to NCAM-120 (present in myelin). The NCAM-180 band appears weaker in tg mice compared to wt controls, in line with the increased polysialylation. (C) Brains were isolated from 4-month-old wt mice and tg littermates (linestg4717 and tg4720) and divided into forebrains (fb), cerebellum (cb) and stem brain (sb). Total homogenates were treated with endoN to remove polySia (+) or left untreated (−). Samples (50 μg of protein/lane) were separated in 10%SDS polyacrylamide gels and transferred onto nitrocellulose membranes. Membranes were probed with rat anti-NCAM antibody H28. Bound primary antibodies were detected with Alexa Fluor 680conjugated anti-rat IgG and an infrared detection system. EndoN-treated samples clearly showed that the total amount of NCAM proteins was not altered in the tg mice when compared to wt littermates. NCAM-180 and NCAM-140 bands were hardly detectable in untreated samples of the tg forebrains, indicating that the majority of NCAM-140 and NCAM-180 proteins carried PSA in tg mice.
Sustained polySia expression in neurons does not affect myelination and myelin maintenance in the CNS
In a first step to assess whether sustained expression of polySia in neurons affects myelination, we collected brains of tg and wt littermates at different developmental stages (P11, P17, P21, and P120) and investigated the expression of myelin basic proteins (MBPs) by immunoblot (Figure 3A). After normalization to beta-actin levels, no significant difference in the amount of MBP (all isoforms were quantified together) was observed between tg mice and wt littermates during the period of active myelination (P11–P21) (Figure 3B). Normal myelin formation in the CNS was confirmed by histological methods using toluidine blue staining (data not shown). To assess whether increased expression of polySia in neurons of adult mice (P120) does not lead to molecular defect of myelin, we investigated the expression level of MBP in the CNS myelin by immunoblot. Results showed no significant difference between tg mice and wt littermates (Figure 3A), confirming the absence of myelin alteration in tg mice. In tg mice overexpressing the polysialyltransferase ST8SiaIV in oligodendrocytes, the ultrastructural analysis of the optic nerve showed a significant alteration of the myelin structure (Fewou et al. 2007). Therefore, we assessed the structure of optic nerve myelin of tg mice overexpressing polySia in neuron by electron microscopy. As shown in Figure 3C tg mice displayed a normal myelin structure when compared to wt littermates. Moreover, myelinated axon tracts of the brain were screened by a modified dark field illumination of free floating sections, visualizing myelinated fibers as dark structures (Weinhold et al. 2005; Hildebrandt et al. 2009). As shown in Figure 4A–N and as discussed in more detail in the next section, the persistent neuronal expression of polySia caused no structural alterations of major myelinated brain fiber tracts. Taken together, these observations suggest that the continuous expression of polySia in neuron does not interfere with myelination, myelin structure and function in the CNS.
Fig. 3.
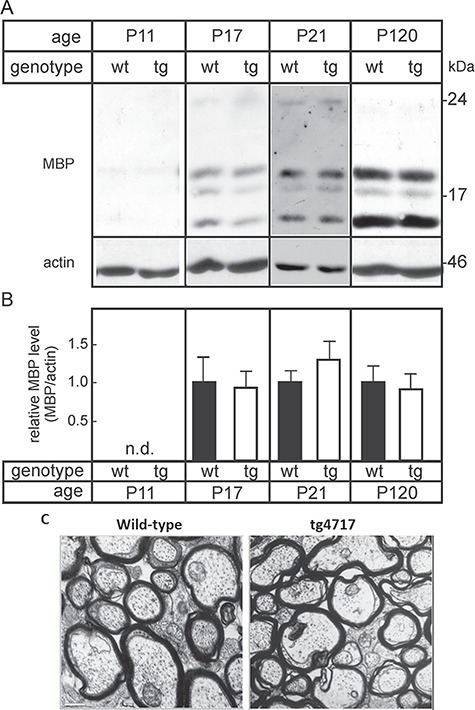
MBP expression in tg and wt mice. Forebrains were isolated from wt mice and tg littermates (line tg4717) at11, 17, 21 and 120 days of age. Total brain membrane fractions were prepared by centrifugation at 100,000 × g, separated (5 μg of protein/lane) in 12% SDS polyacrylamide gels and transferred onto nitrocellulose membranes. (A) Membranes were probed with antibodies against MBP and actin. (B) MBP bands were quantified by densitometry and normalized to actin protein levels. Mean MBP levels in wt brains were set to 100%. Columns indicate mean +/− SEM (n = 3 for P17and P120; n = 5 for P21) (n.d., not done). No significant differences between tg and wt mice were observed (t-test). (C) Electron micrographs of cross sections of the optic nerve did not show any obvious difference in the degree of myelination and myelin structure between tg mice (A) and wt littermates (B). Scale bar, 0.6 μm.
Fig. 4.
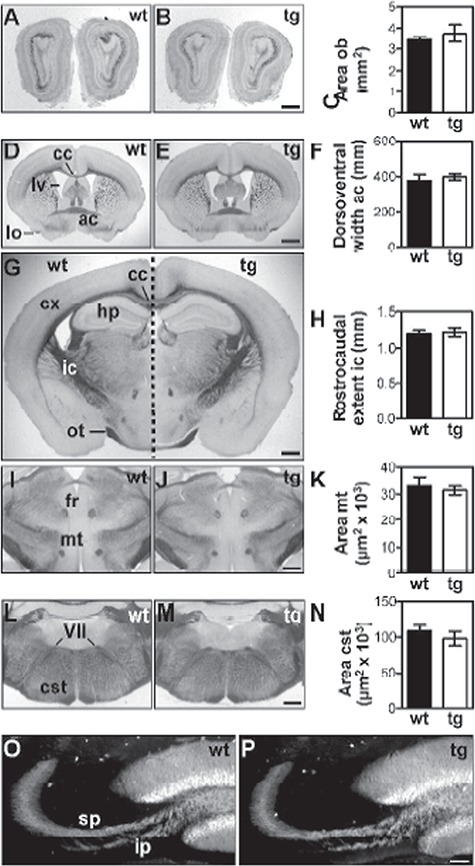
Brain morphology of ST8SiaIV-tg mice. Coronal sections through brains from 31 days old female wt (left panels) and tg mice (right panels). Unstained vibratom sections under modified dark field illumination illustrate normal size of obs (B) and lv (E), normal thickness of the cx (E, G) as well as normal position and morphology of the hippocampal formation (hp; G) and of major fiber tracts in the tg mice. The size of the obs (A–C) and of selected fiber tracts, i.e. ac (D–F), ic (G, H), mt (I–K) and cst at the level of the seventh cranial nerve (VII; L–N) were evaluated by morphometry (graphs in right column). Other fiber tracts depicted comprise the lateral olfactory tract (lo; D), corpus callosum (cc; D, G), optic tract (ot; G) and fasciculus retroflexus (fr; I). Immunofluorescent staining with calbindin-specific antibodies was used to visualize normal fasciculation and lamination of the mossy fiber tract into the supra and infrapyramidal bundel (sp, ip) in wt littermates (O) and ST8SiaIV-tg mice (P). Data in graphs represent mean values +/− s.d. obtained from four wt controls and three tg animals, each. Statistical evaluation revealed no significant difference between each pair of means (two-tailed t-test, P > 0.1). Scale bars, 1 mm (E), 500 μm (B, G, J, M) and 100 μm (P).
Normal brain morphology of ST8SiaIV-tg mice
ST8SiaIV-tg mice were born at a normal Mendelian frequency, and no inexplicable death was recorded in the tg mouse population compared to wt littermates. In all of the phenotypic analyses performed, tg mice and wt controls were indistinguishable at birth and throughout development. Moreover, tg mice showed no macroscopically visible abnormalities such as body shape and weight. In order to identify a potential developmental defect in the brain due to a sustained expression of polySia in neurons, we focused our effort on development of the forebrain, which displays a substantial down-regulation of polySia during postnatal development (Hildebrandt et al. 2007) and analyzed brain structures that are known to be particularly affected in the absence of polySia (Weinhold et al. 2005; Angata et al. 2007; Hildebrandt et al. 2009). The most obvious morphological aberrations found in NCAM- and polySia-deficient as well as in polysialyltransferase-negative mice comprise a dramatic size reduction of the obs, a delamination of the mossy fiber tract in the hp formation and hypoplasia of the cst. Analyses of these parameters showed no significant differences between tg mice and their wt littermates (Figure 4A–C, ob; Figure 4G, hp; Figure 4L–N, cst). Moreover, the size of the ac (Figure 4D-F, ac), the ic (Figure 4G, H, ic) and the mammillothalamic tract (mt) [Figure 4I–K, mt), which are affected in mice with imbalanced NCAM and polySia levels, were not altered in ST8SiaIV tg mice, and the size of the lateral ventricles (lvs) and the thickness of the cerebral cortex (cx) were similar in all analyzed tg and wt littermates (Figure 4D–G). Finally, immunofluorescence with calbindin-specific antibody revealed normal fasciculation and absence of delamination of mossy fibers (Figure 4O, P). Collectively, these data suggest that a sustained increase of polySia in neurons does not affect brain development and architecture.
Motor coordination in ST8SiaIV tg mice
To measure motor-coordinative capacities tg (line tg4717) and age-matched wt mice were tested in a rotating rod experiment (Figure 5A). Mice were trained to stay on the rod rotating at 3 rpm for 60 seconds for 3 d. On the fourth day, testing was done at four different speeds (3, 6, 18 and 24 rpm), and the number of cumulative falls within 60 seconds was determined. The number of cumulative falls increased with rotarod speed, as expected, however, there was no difference between tg mice and wt littermates. These results indicate that mice overexpressing polySia in neurons have no impairment in motor coordination.
Fig. 5.
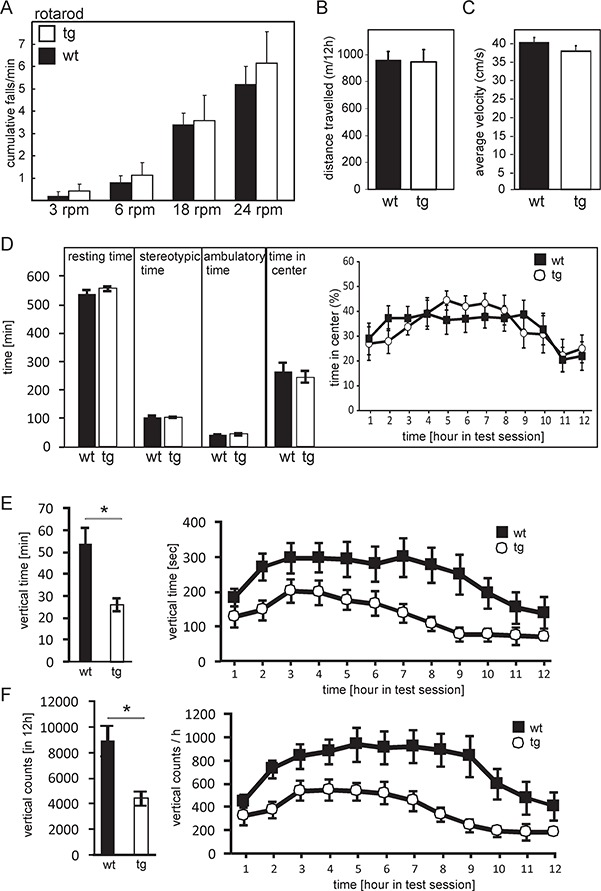
Open field and rotarod test. (A) In the rotarod assay, female mice (7–8 months old) (wt: n = 5; tg: n = 7) trained as described in the experimental procedures section, were tested at increasing speeds in a rotarod apparatus and the number of cumulative falls within 1 min was recorded. No significant differences were observed between tg mice and wt littermates. For the open field test (B–F), female tg mice of the line tg4717 (n = 9) and wt littermates (n = 7) (7–8 months of age) were monitored in an open field box (area: 44 × 44 cm) for 12 h during the dark period of the 12 h/12 h light/dark cycle. Data analysis indicated no significant differences in the total activity (distance traveled) (B), average velocity (C), resting time, sterotypic time, ambulatory time or time spent in the center of the test area (D). In contrast, rearing activity, measured as vertical time (E; *P = 0.0019) and vertical counts (F; *P = 0.0035) showed a significant effect of genotype (ANOVA with post hoc Bonferroni test), with a strong reduction of activity in tg mice. All data shown are the mean ± SEM.
Mild deficit of exploratory behavior in polysialyltransferase ST8SiaIV tg mice
The open field is a standard test used to determine differences in locomotor activity, exploratory behavior and anxiety between two groups of animals genetically different or pharmacologically treated (Hall, 1934; Wilson et al. 1976; Walsh and Cummins, 1976; Stanford, 2007; Seibenhener and Wooten, 2015). The open-field investigation is reduced to study the exploratory behavior of an animal when during the test the animal is introduced in the arena for a short period of time (Fonio and Golani 2012; Fonio and Benjamini 2012; Wexler et al. 2018). Moreover, a normal behavior of an animal in an open-field arena is to migrate to the periphery seeking for protection in contrast to being vulnerable at the center of the arena. Therefore, anxious animals spend less time in the center of the arena (Belzung and Griebel, 2001; Prut and Belzung 2003; Ojha et al. 2011). To investigate possible changes in locomotor activity, anxiety and exploratory behavior due to sustained expression of polySia in neurons, we subjected adult, 7- to 8-months-old, female tg (line tg4717, n = 9) and wt control littermates (n = 7) to open-field analysis. Mice were placed in the center of the open field, and their movement was recorded for 12 h. Overall distance traveled (Figure 5B), average velocity (Figure 5C) and the time period spent with a specific movement pattern (resting, stereotypic ambulatory, time spend in the center) (Figure 5D) were assessed. In addition, vertical time and counts were monitored (Figure 5E, F). tg mice and wt littermates showed no differences in overall distance traveled (Figure 5B), mean velocity (Figure 5C), resting, stereotypic and ambulatory (Figure 5D). Time spent in the center of the open-field arena, a measure of anxiety, was also not significantly different between wt and tg mice (Figure 5D). Analysis of time-depending changes in the center time showed significant alterations across time bins (ANOVA; effect of genotype P = 0.002), as expected (Fonio and Golani 2012; Fonio and Benjamini 2012), but no significant differences between genotypes (P = 0.944), indicating normal anxiety-related behavior in the tg mice (Figure 5D). However, tg mice displayed a significant reduction of vertical activity time (P = 0.0019) and counts (P = 0.0035) (rearing), a measure for exploratory behavior (Figure 5E, F). Rearing activity changed significantly over time in both genotypes (effect of time P < 0.0001) but there was no genotype × time interaction (P = 0.878). Taken together, these data suggest that tg mice exhibit largely a normal locomotor and anxiety-like behavior, but display reduced exploratory activity.
Discussion
The current study was undertaken according to the general hypothesis that a sustained expression of polySia in neurons affects the nervous system development and functioning and to complement experimental findings that permanent expression of polySia in oligodendrocytes leads to hypo-myelination and myelin instability (Fewou et al. 2007). Here we demonstrate that expression of the polysialyltransferase ST8SiaIV under the control of the neuron-specific Thy1.2promoter prevents the postnatal downregulation of polySia. Unlike the forced expression ofST8SiaIV in oligodendrocytes, which resulted in an increase of polySia concentration on NCAM-120 (Fewou et al. 2007), the use of Thy1.2 promoter in the tg construct resulted in the prevention of the down-regulation of polySia on NCAM-140 and NCAM-180 in the brain of tg mice. In addition, no delay of developmental myelination as well as impaired myelin structure was observed in tg mice. Likewise, no neuroanatomical and cognitive deficiency was observed in tg mice. Together, the data indicate that permanent expression of polySia in neuron does not affect the brain development and functioning. However, permanent polysialylation of neuronal (axonal) membrane induce a mild behavioral deficit.
The NCAM belongs to the superfamily of immunoglobulin-like surface molecules. The two major isoforms NCAM-140 and NCAM-180 in neuron have identical extracellular domains and are both transmembrane glycoproteins. In addition to several extracellular interactions, NCAM can undergo interactions with various cytosolic binding partners and activates several signal transduction pathways (Büttner et al. 2003; Povlsen et al. 2003; Walmod et al. 2004; Büttner et al. 2005). Polysialylation of these proteins therefore modulate their functions. Indeed, most NCAM-140 and NCAM-180 were polysialylated beyond adulthood in the brain of tg mice. Since NCAM is not the sole polySia carrier in mammalian brain (Mühlenhoff et al. 1996; Curreli et al. 2007; Galuska et al. 2010; Mühlenhoff et al. 2013), we cannot rule out that the phenotype observed in polysialyltransferase ST8SiaIV overexpressing mice may be due to the gain of polySia by other cell adhesion molecules or non-NCAM protein present in neuron or another cell type. However, the sustained expression of polySia on NCAM-140 and NCAM-180 had no effect on any of the other evaluated parameters of brain morphology. Nonetheless, behavioral investigations showed a significant reduction of vertical activity. Taken together, the results of the current study indicate that preventing the downregulation of polySia in the brain by forced expression of ST8SiaIV in neurons has no detectable effect on brain morphology and myelination, but causes a mild behavioral phenotype.
Our behavioral studies indicate a significant decrease in rearing activity of ST8SiaIV-tg mice in the open-field test, which is thought to reflect exploratory behavior and motor activity (Archer, 1973). Because total activity, time spent in the center of the open-field arena and other parameters tested in the open-field test were not affected, reduced rearing activity in theST8SiaIV-tg mice is most likely to be attributed to deficits in exploratory behavior and not to reduce anxiety. Thus, the lower exploratory drive of ST8SiaIV-tg mice may be a consequence of a sustained expression of polySia on NCAM-140/180 in neurons. The specific reduction in rearing activity may suggest a selective impairment of exploratory activity toward more distant (visual) stimuli, perceivable only upon head lifting. Rearing may, however, also simply indicate an escape reaction (Lever et al. 2006). The available data do currently not allow to deciding which of these behavioral activities is affected in ST8SiaIV tg mice.
The fact that severe developmental defects in mice lacking polySia can be rescued by additional deletion of the NCAM gene (Weinhold et al. 2005) has shown that polySia is important as a negative regulator of NCAM function. Therefore, behavioral changes caused by sustained expression of polySia on NCAM could mimic loss of specific NCAM function. In a different (not open-field based) test system NCAM-deficient mice showed reduced exploratory activity or escape reaction (Cremer et al. 1994). However, Stork et al. (1999) found no significant changes in rearing activity in NCAM-deficient mice on a C57BL/6 background (corresponding to the genetic background of the tg mice reported here) and increased rearing activity on a 129/Ola genetic background. Reduced rearing activity in the absence of neuroanatomical defects has also been described in tg mice with overexpression of a dominant-negative form of cadherin using α-CaMK II-Cre mice, blocking functions of classical cadherins in cortex and hippocampus (Edsbagge et al. 2004). Notably, cell interaction studies and molecular force measurements demonstrated that repulsion by polySia is sufficient to overwhelm not only homophilic NCAM interactions but also cadherin attraction at physiological ionic strength (Johnson et al. 2005; Fujimoto et al. 2001), giving rise to the possibility that interference with cadherin functions is the mechanism how enhanced polysialylation in neurons as achieved by the ST8siaIV-tg mice affects rearing activity. This is currently under investigation.
The role of axonal polySia in regulating myelination is not fully understood. Enzymatic removal of polySia using endoneuraminidase increased the number of myelinated internodes in vitro and in vivo (Charles et al. 2000). Though this treatment removes polySia from all cells, the data reported by Charles et al. (2000) suggested that the removal of polySia from axons was responsible for the observed effect. Meyer-Franke et al. (1999) reported that removal of polySia from axonal membranes in neuron-oligodendrocyte co-cultures induced the alignment of oligodendrocytes to axons. Inhibition of oligodendrocyte-axon interactions in the presence of polySia (Meyer-Franke et al. 1999) could be due to steric hindrance by the large polySia molecule. These results led to the hypothesis that down-regulation of axonal polySia is necessary for an efficient myelination and suggested that the transcriptional down-regulation of polysialyltransferases observed during postnatal development is a necessary prerequisite for myelination. To test this hypothesis in vivo, we previously generated tg mice that maintain oligodendrocytic polySia beyond the phase of myelination. Analysis of these mice suggested that loss of polySia on oligodendrocytes is not an absolute requirement for myelination, but down-regulation of polySia at the end of oligodendrocytes precursor cell differentiation is important to avoid delay in the process of myelin formation (Fewou et al. 2007; Werneburg et al. 2017). In order to discriminate between oligodendrocytic and neuronal polySia, we now generated mice with sustained expression of polySia in neurons. Although these mice showed a significant expression of polySia on axons maintained into adulthood as assessed by immunoblot of optic nerve, spinal cord and brain, we could not observe any alterations of myelination. In addition, the myelin structure and thickness were unaltered in the tg mice. The current data demonstrate that down-regulation of polySia of neuronal origin during postnatal brain development is not a prerequisite for building the gross anatomy of the brain, including efficient myelination and morphology of major brain fiber tracts. In addition, we can conclude that “contrasting” the myelin deficits of PLP-ST8SiaIV tg mice with the absence of neurodevelopmental defects in tg mice over-expressing polySia at adult age provide the first in vivo evidence for different consequences of neuronal and oligodendrocytic dysregulation of polySia.
In the current study, we have shown that preventing the developmental downregulation of polySia in neurons caused no apparent defects of brain morphology. Myelination and myelin structure in the CNS of tg mice were not altered, indicating that in contrast to the reduction of polySia on oligodendroctyes (Fewou et al. 2007), down-regulation of neuronal polySia is not a prerequisite for efficient myelin formation. In addition, sustained expression of polySia in neurons was compatible with normal brain development and architecture. However, reduced rearing was observed in the open-field test. This could be caused by a dysfunction of the hippocampus due to increased polySia levels, since the regulation of cell adhesion in the hippocampus appears to be crucial for controlling rearing in a novel environment (Edsbagge et al. 2004; Lever et al. 2006). Overall, the absence of a neurodevelopmental phenotype qualifies the Thy1-ST8SiaIV tg mice as a useful model system to further explore the effects of a gain of polySia on neuroplasticity of the mature brain.
Materials and methods
Animals
This research study was not pre-registered. tg C57BL/6J mice expressing the mouse polysialyltransferase ST8SiaIV under the control of the Thy1.2 promoter were generated at the Karolinska Center for Transgene Technologies (Stockholm, Sweden). Mice were housed as a group of 3–5 in a tinted open top mouse cage and were maintained under conditions of controlled temperature and lighting (light/dark cycle: lights on, 06:00; lights off, 18:00). The animals had ad libitum access to food and water. All animal experiments were approved by the University of Bonn Animal Ethics Committee and performed in accordance with the guidelines of the Animal Care and Experimentation Committee of the University of Bonn. All efforts were made to minimize the number of animals used and to minimize suffering. A minimum of five female tg mice and wt controls where used for biochemical and histological analysis. Female tg mice (nine) and wt littermates (seven females) where used for behavioral study.
Generation of polysialyltransferase ST8SiaIV-tg mice
To generate the tg animals, the mouse polysialyltransferase ST8SiaIV was amplified by polymerase chain reaction (PCR) using oligonucleotides primers PSTsense (5′-GCAAGCTTCCATGCGCTCAATTAGAAAACG-3′) and PSTanti (5′-GCTCTAGATTATTGCTTCATGCACTTTCC-3′), introducing HindIII and XbaI sites. The amplified cDNA was then digested with HindIII and XbaI, treated with Klenow enzyme (fill-in reaction) and ligated into the Thy1.2 promoter cassette (kindly provided by Herman van der Putten, Novartis Pharma Inc., Basel, Switzerland; van der Putten et al., 2000) (see Figure 1A) preliminarily treated with XhoI and Klenow enzymes. Correct sequence of ST8SiaIV cDNA was confirmed by DNA sequencing. In order to generate tg mice, vector sequences were removed from the plasmid by digesting the DNA with NotI, followed by agarose gel separation. The transgene fragment was extracted from the gel by electroelution in dialysis bags. The DNA fragment was finally purified using Elutip (Schleicher & Schuell, Dassell, Germany). Pronucleus injection into fertilized eggs from C57BL/6 mice was performed at the Karolinska Center for Transgene Technologies (Stockholm, Sweden). Founder mice were analyzed by Southern blotting with the [32P]-labeled ST8SiaIV cDNA coding sequence as the probe using standard procedures (Sambrook et al. 1989). Several tg founder mice were obtained, from which two independent tg lines (tg4717 and tg4720) were established and maintained on an inbred C57BL/6genetic background. Genotyping of tg mice was done by PCR on genomic tail DNA using the sense (5′-CCATGCGCTCAATTAGAAAACG-3′) and antisense (5′-GCTCTAGATTATTGCTTCATGCACTTTCC-3′) oligonucleotides, resulting in a product of 1091 base pair in the presence of ST8SiaIV transgene. PCR conditions were as follows: 94°C × 5 min, followed by 32 cycles of 94°C × 30 seconds, 56°C × 30 seconds, 72°C × 60 seconds.
Northern blot analysis
For northern blot analysis, tg mice and wt littermates were killed by cervical dissociation and the isolated mouse brain frozen in liquid nitrogen. Brain RNA was isolated byCsCl gradient centrifugation as described (Sambrook et al., 1989). Total RNA (20 μg) was separated in 1% agarose formaldehyde gels and transferred onto Hybond-N+ nylon membranes (Amersham Biosciences, Freiburg, Germany). A ST8SiaIV cDNA probe (HindIII-XbaI fragment of the tg construct used for generating the tg mice) was generated with [α-32P] dCTP by random-priming using Megaprime DNA Labeling System (Amersham Biosciences, Freiburg, Germany) following the instructions of the manufacturer. Hybridization was done as described (Fewou et al. 2005). Membranes were exposed to Bio-Imager screens followed by exposure to X-ray films.
In situ hybridization
The mouse polysialyltransferase ST8SiaIV was subcloned into the pcDNA3 (RRID:Addgene_42208) vector and use as a template for the synthesis of digoxigenin-11-labeled (Roche Molecular Diagnostics, Mannheim, Germany) antisense and sense probes cRNA probe. tg mice and wt littermates were anaesthetized using 2.5% tribromoethanol (Fluka,Germany) and perfused with 4% paraformaldehyde (PFA). Organs were isolated and postfixed, washed and 12 μm frozen sections generated. In situ hybridization on retina, spinal cord and brain sagittal cryosections (12 μm) was done as recently described (Fewou et al. 2005). Briefly, frozen slice were fixed and hybridization was performed using the appropriate Digoxigenin-11-UTP-labeled antisense or sense cRNA probes at 65°C in 50% formamide, 1% Denhardt’s solution, 0.2% SDS, 0.25 mg/mL ssDNA, 0.25 mg/mL tRNA and 10% hybridization salt (3 M NaCl, 0.1 MPIPES, 0.1 M EDTA) overnight in humid chamber. Unspecific bound were removed by washes in 2X SSC and 0.1X SSC successively. Bound probes were visualized with alkaline phosphatase-conjugated anti-digoxigenin antibody (Roche Molecular Diagnostics Cat# 11093274910,RRID:AB_514497) and nitro blue tetrazolium/bromo-chloro-indolyl phosphate as substrate. Sections were examined with a Zeiss Axiovert inverted microscope (Carl Zeiss, Jena, Germany).
Membrane preparation and endoneuraminidase treatment
tg mice and wt littermate were killed by cervical dissociation. Brains or nerve samples were homogenized in 20 mM Tris-HCl (pH 7.4), 150 mM NaCl (TBS), 5 mM EDTA, 1 mM PMSF using an Ultraturrax homogenizer (Janke & Kunkel, Staufen, Germany). Homogenates were centrifuged at 1000 × g for 10 min, and the supernatants were centrifuged again at 100,000 × g for 1 h (4°C) to isolate membranes. The pellet was re-suspended in TBS. Protein concentrations were determined using the Biorad DC assay (Biorad, München, Germany). In order to specifically remove polySia, protein samples were treated with endoN (Mühlenhoff et al. 2003; kindly provided by Rita Gerardy-Schahn, Medizinische Hochschule Hannover, Germany) using 10 μg/mL endoN at 25°C for 60 min.
SDS-PAGE and western blotting
For SDS-PAGE and western blotting, tg mice and wt littermates were killed by cervical dissociation. Brains from tg and wt littermates were homogenized in protein extraction buffer (20 mM Tris-HCl pH 8.0, 5 mM EDTA, 1 mM PMSF, 20 μg/mL aprotinin, 1 μg/mL leupeptin, 50 mM NaCl, 0.5% SDS) and SDS-PAGE in 7%, 10% and 12%polyacrylamide gels was done using standard procedures (Harlow and Lane, 1988). Proteins were then transferred onto nitrocellulose membranes (pore size 0.45 μm; Schleicher & Schuell, Dassell, Germany) using a semi-dry blotting system and a transfer buffer (48 mM Tris, 39 mM glycine) as described (Harlow and Lane, 1988). To block non-specific binding sites, 3% (w/v) nonfat dry milk in TBS was used, and antibodies were diluted in the same buffer. NCAM (H28) (1/200) (GeneTex Cat# GTX19782, RRID:AB_423730), polySia (735) (2.5 μg/mL) (Dr. Muhlenhoff Medizinische Hochschule Hannover Cat# mab-735, RRID:AB_2619682), rabbit anti-MBP (1/5000) antiserum (Millipore Cat# AB5864, RRID:AB_2140351) and mouse anti-actin IgG (1/1000) (Sigma-Aldrich Cat# A2172, RRID:AB_476695) in blocking solution. To remove antibodies non-specifically bound to membrane, a TBS buffer containing 0.3% Tween-20 was used. To detect bound primary antibodies, the appropriate peroxidase-conjugated secondary antibodies (Dianova Cat# 115-036-003, RRID:AB_2617176; Jackson Immuno Research Labs Cat# 111–036-006, RRID:AB_2313586; Rockland Cat# 812-1302, RRID:AB_218917) followed by chemiluminescence detection was used as described (Eckhardt et al. 2002). In some experiments western blots were developed using Alexa Fluor 680 goat anti-rat IgG (Molecular Probes Cat# A-21096, RRID:AB_141554) and IR Dye 800CW goat anti-mouse IgG (Rockland Cat# 610-131-003,RRID:AB_220122) as secondary antibodies and an infrared detection system (LI-COR infrared scanning system; LI-COR Biosciences, Bad Homburg, Germany), as described (Zöller et al. 2005). Quantification of signal intensities by densitometry was made using AIDA software (AIDA Tookit;RRID:SCR_005914) (Raytest, Straubenhardt, Germany). Student’s t-test was used for statistics.
Histology and morphometry
For histological and morphometric analysis, female tg mice and wt littermates of 31 days of age were deeply anesthetized with 2,2,2-tribromoethanol (15 mL/kg of2.5% 2,2,2-tribromoethanol in 0.9% NaCl). Transcardial perfusion was performed with 4% PFA before brains were removed and postfixed overnight. Brains were further embedded in 4% agar-agar (Roth, Germany) and cut into 50 μm serial sections using a vibrating microtome. Floating sections were examined using a stereomicroscope (Zeiss Discovery) under modified dark field illumination. Micrographs were archived digitally using AxioVision software (RRIB: SCR_002677). The rostrocaudal extent of the ic was calculated as the product of the number of coronal sections on which the ic was present multiplied by the thickness of the sections (50 μm). Moreover, area and length measurements were performed on digital images using ImageJ software (ImageJ, RRID:SCR_003070). The cross-sectional areas of the ob as well as of the cst and mt were measured as illustrated in the respective figures (see Results), and for each animal the mean values of left and right structures were calculated. The dorsoventral width of the ac was measured perpendicular to its trajectory at the level of midline crossing on coronal serial sections. Statistical analyses were performed using Graphpad Prism software (RRID:SCR_002798).
Immunohistochemistry
For Immunofluorescence staining, 2,2,2-tribromoethanol-anesthetized adult female tg mice and wt littermates were perfused with 4% PFA via the left cardiac ventricle. Brains were removed from the scalp and postfixed overnight in 4% PFA and finally washed. Floating vibratome sections generated from the brain of tg mice and wt littermates was labeled with rabbit polyclonal anti-calbindin antibody (Swant, 1/200, Cat# CB 38, RRID:AB_10000340) and Alexa Fluor 488-conjugatedsecondary antibodies (Molecular Probes, 1/500, Cat# A-11070, RRID:AB_142134) according to the manufacturers’ instructions. Antibodies were diluted in PBS containing 5% bovine serum. All sections were coverslipped using Vectashield mounting medium with DAPI (Vector Laboratories Cat# H-1200, RRID:AB_2336790). Microscopy was performed using a Zeiss Axiovert 200Mequipped with AxioCam MRm digital camera and AxioVison software (RRID: SCR_002677).
Electron microscopy
Light and electron microscopy analysis were performed as described (Büssow 1978; Uschkureit et al. 2000). Briefly, 2,2,2-tribromoethanol-anesthetized adult tg mice and wt littermates from the line 4717 were perfused with 6% glutaraldehyde via the left cardiac ventricle. The optic nerves were isolated and post fixed in phosphate-buffered osmium tetroxide (OSO4) in sucrose, and embedded using Epon 812. The semi thin sections generated from the embedded optic nerves were stained with toluidine/pyronin and the ultra-thin sections were contrasted with uranyl acetate and lead citrate and examined as described (Büssow, 1978).
Rotarod test
Rotarod tests were done as described by Kuhn et al. (1995). Briefly, Female mice at 7–8 months of age (n = 9 for tg mice of the line tg4717 and n = 7 for wt littermates controls) were accustomed to the rotarod apparatus by placing them on the rotating rod and the rod rotation accelerated to a rate of 3 rpm for 10 seconds on three consecutive days. On the fourth day, mice were placed on the rod and the rod rotation accelerated to a rate of 3 rpm. The experiments were repeated at 6, 18 and 24 rpm and the number of cumulative falls within 1 min was recorded (timing was stopped every time a mouse fell off the rod). The experimentator was blinded to the identity of the mouse tested. Statistical analyses were performed using Graphpad Prism software (RRID: SCR_002677) and groups were tested for significance using ANOVA.
Open field test
Female mice, 7–8-month-old (nine tg mice of the line tg4717 and seven wt littermates) were placed in the center of the chamber of the open field apparatus made of transparent plexiglass cage (44 × 44 × 30 cm) and movements were monitored with an automatic monitoring system from MED associates (RRID:SCR_014296) for 12 h during the dark period of the 12/12 h light/dark cycle. Distance traveled, average velocity, resting time, stereotypic time, ambulatory time, time spend in center of the arena, vertical time and counts were recorded. Data were analyzed for each hour over a total period of 12 h using activity software (RRID:SCR_014296) and tested for significance using ANOVA.
Data analysis and statistics
To avoid any kind of bias during the investigation, the experimentator was blinded to the identity of the mice or samples under analysis. For statistical analyses, GraphPad Prism (RRID:SCR_002677) was used and also for the presentation of bar graphs. To quantitatively compare data from tg mice and wt littermates, normality was first assessed in order to choose the type of statistic to be used. To compare mean value from tg mice and wt littermates, ANOVA followed by post hoc Bonferroni test was performed. Statistical significances were set to P < 0.05. Errors are shown throughout the manuscript as standard error of the mean (SEM). Figures montages were made using Microsoft power point software (Microsoft Corporation, Washington, USA).
Funding
Deutsche Forschungsgemeinschaft (grant number EC164/2 to M.E. and Hi678/8-1 to H.H.).
Author contributions
M.E. conceived and coordinated the study, participated in the data collection and interpretation and manuscript writing; S.N.F. generated the tg mice, participated in the data collection and interpretation and wrote the manuscript; I.R. participated in the data collection; H.H. participated in data collection and interpretation and manuscript writing.
Acknowledgements
We thank Heinrich Büssow for electron microscopy and Ivonne Becker, Natascha Heidrich and Hannelore Burkhardt for technical assistance. We further thank Herman van der Putten for the Thy1.2 promoter construct and Rita Gerardy-Schahn for kindly providing735 antibody and endoneuraminidase.
Abbreviations
- APS, ammonium persulfate
-
CNS, central nervous system
CsCl, cesium chloride
DAPI, 4',6-diamidino-2-phenylindole
EDTA, ethylenediaminetetraacetic acid
endoN, endoneuraminidase
MBP, myelin basic protein
NCAM, neural cell adhesion molecule
OPC, oligodendrocyte precursor cell
PCR, polymerase chain reaction
PLP, proteolipid protein
PMSF, phenylmethylsulfonyl fluoride
PolySia, polysialic acid
SDS, sodium dodecyl sulfate
ST8SiaII, ST8 Alpha-N-Acetyl-Neuraminide Alpha-2, 8-Sialyltransferase 2
ST8SiaIV, ST8 Alpha-N-Acetyl-Neuraminide Alpha-2, 8-sialyltransferase 4
TBS, tris buffered saline
Conflict of interest statement
None declared.
References
- Alves R, Barbosa de Carvalho JG, Venditti MAC. 2012. High- and low-rearing rats differ in the brain excitability controlled by the allosteric benzodiazepine site in the GABA A receptor. J Behav Brain Sci. 2:315–325. [Google Scholar]
- Angata K, Fukuda M. 2003. Polysialyltransferases: Major players in polysialic acid synthesis on the neural cell adhesion molecule. Biochimie. 85:195–206. [DOI] [PubMed] [Google Scholar]
- Angata K, Huckaby V, Ranscht B, Terskikh A, Marth JD, Fukuda M. 2007. Polysialic acid-directed migration and differentiation of neural precursors are essential for mouse brain development. Mol Cell Biol. 27:6659–6668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angata K, Long J-M, Bukalo O, Lee W, Dityatev A, Wynshaw-Boris A, Schachner M, Fukuda M, Marth J. 2004. Sialyltransferase ST8Sia-II assembles a subset of polysialic acid that directs hippocampal axonal targeting and promotes fear behavior. J Biol Chem. 279:32603–32613. [DOI] [PubMed] [Google Scholar]
- Archer J. 1973. Test for emotionality in rats and mice. Animal Behav. 21:205–235. [DOI] [PubMed] [Google Scholar]
- Belzung C, Griebel G. 2001. Measuring normal and pathological anxiety-like behaviour in mice: A review. Behav Brain Res. 125:141–149. [DOI] [PubMed] [Google Scholar]
- Bercury KK, Macklin WB. 2015. Dynamics and mechanisms of CNS myelination. Dev Cell. 32:447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier PJ, Bedard A, Vinet J, Levesque M, Parent A. 2002. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc Natl Acad Sci U S A. 99:11464–11469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfanti L. 2006. PSA-NCAM in mammalian structural plasticity and neurogenesis. Prog Neurobiol. 80:129–164. [DOI] [PubMed] [Google Scholar]
- Büttner B, Kannicht C, Reutter W, Horstkorte R. 2003. The neural cell adhesion molecule is associated with major components of the cytoskeleton. Biochem Biophys Res Comm. 310:967–971. [DOI] [PubMed] [Google Scholar]
- Büttner B, Kann nicht C, Reutter W, Horstkorte R. 2005. Novel cytosolic binding partners of the neural cell adhesion molecule: Mapping the binding domains of PLC gamma, LANP, TOAD-64, syndapin, PP1, and PP2A. Biochemistry. 44:6938–6947. [DOI] [PubMed] [Google Scholar]
- Camand E, Morel MP, Faissner A, Sotelo C, Dusart I. 2004. Long-term changes in the molecular composition of the glial scar and progressive increase of serotoninergic fibre sprouting after hemisection of the mouse spinal cord. Eur J Neurosci. 20:1161–1176. [DOI] [PubMed] [Google Scholar]
- Charles P, Hernandez MP, Stankoff B, Aigrot MS, Colin C, Rougon G, Zalc B, Lubetzki C. 2000. Negative regulation of central nervous system myelination by polysialylated-neural cell adhesion molecule. Proc Natl Acad Sci U S A. 97:7585–7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles P, Reynolds R, Seilhean D, Rougon G, Aigrot MS, Niezgoda A, Zalc B, Lubetzki C. 2002. Re-expression of PSA-NCAM by demyelinated axons: An inhibitor of remyelination in multiplesclerosis. Brain. 125:1972–1979. [DOI] [PubMed] [Google Scholar]
- Cremer H, Lange R, Christoph A, Plomann M, Vopper G, Roes J, Brown R, Baldwin S, KraemerP SS, Barthels D et al. . 1994. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature. 367:455–459. [DOI] [PubMed] [Google Scholar]
- Crusio WE. 2001. Genetic dissection of mouse exploratory behavior. Behav Brain Res. 125:127–132. [DOI] [PubMed] [Google Scholar]
- Curreli S, Arany Z, Gerardy-Schahn R, Mann D, Stamatos NM. 2007. Polysialylated neuropilin-2is expressed on the surface of human dendritic cells and modulates dendritic cell-T lymphocyte interactions. J Biol Chem. 282:30346–30356. [DOI] [PubMed] [Google Scholar]
- Decker L, Avellana-Adalid V, Nait-Oumesmar B, Durbec P, Baron-Van Evercooren A. 2000. Oligodendrocyte precursor migration and differentiation: Combined effects of PSA residues, growth factors, and substrates. Mol Cell Neurosci. 16:422–439. [DOI] [PubMed] [Google Scholar]
- Decker L, Durbec P, Rougon G, Evercooren AB. 2002. Loss of polysialic acid residues accelerates CNS neural precursor differentiation in pathological conditions. Mol Cell Neurosci. 19:225–238. [DOI] [PubMed] [Google Scholar]
- Eckhardt M, Fewou SN, Ackermann I, Gieselmann V. 2002. N-glycosylation is required for full enzymic activity of the murine galactosylceramide sulphotransferase. Biochem J. 368:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edsbagge J, Zhu S, Xiao MY, Wigström H, Mohammed AH, Semb H. 2004. Expression of dominant negative cadherin in the adult mouse brain modifies rearing behavior. Mol Cell Neurosci. 25:524–535. [DOI] [PubMed] [Google Scholar]
- Fewou SN, Büssow H, Schaeren-Wiemers N, Vanier MT, Macklin WB, Gieselmann V, Eckhardt M. 2005. Reversal of non-hydroxy:alpha-hydroxy galactosylceramide ratio and unstable myelin in transgenic mice overexpressing UDP-galactose:ceramide galactosyltransferase. J Neurochem. 94:469–481. [DOI] [PubMed] [Google Scholar]
- Fewou SN, Ramakrishnan H, Büssow H, Gieselmann V, Eckhardt M. 2007. Down-regulation of polysialic acid is required for efficient myelin formation. J Biol Chem. 282:16700–16711. [DOI] [PubMed] [Google Scholar]
- Finne J, Finne U, Deagostini-Bazin H, Goridis C. 1983. Occurrence of α-2-8 linked polysialosyl units in a neural cell adhesion molecule. Biochem Biophys Res Comm. 112:482–487. [DOI] [PubMed] [Google Scholar]
- Fonio E, Golani I, Benjamini Y. 2012a. Measuring behavior of animal models: Faults and remedies. Nat Methods. 9:1167–1170. [DOI] [PubMed] [Google Scholar]
- Fonio E, Benjamini Y, Golani I. 2012b. Short and long term measures of anxiety exhibit opposite results. PLoS One. 7: e48414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto I, Bruses JL, Rutishauser U. 2001. Regulation of cell adhesion by polysialic acid. Effects on cadherin, immunoglobulin cell adhesion molecule, and integrin function and independence from neural cell adhesion molecule binding or signaling activity. J Biol Chem. 276:31745–31751. [DOI] [PubMed] [Google Scholar]
- Gage FH. 2000. Mammalian neural stem cells. Science. 287:1433–1438. [DOI] [PubMed] [Google Scholar]
- Galuska SP, Rollenhagen M, Kaup M, Eggers K, Oltmann-Norden I, Schiff M, Hartmann M, Weinhold B, Hildebrandt H, Geyer R et al. . 2010. Synaptic cell adhesion molecule SynCAM1 is a target for polysialylation in postnatal mouse brain. Proc Natl Acad Sci USA. 107:10250–10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascon E, Vutskits L, Kiss JZ. 2007. Polysialic acid-neural cell adhesion molecule in brain plasticity: From synapses to integration of new neurons. Brain Res. 56:101–118. [DOI] [PubMed] [Google Scholar]
- Hall CS. 1934. Emotional behaviour in the rat. I. Defecation and urination as measures of individual differences in emotionality. J Compar Psychol. 18:385–403. [Google Scholar]
- Harlow E, Lane D. 1988. Antibodies. A Laboratory Manual. Cold Spring Harbor, NY: Cold SpringHarbor Laboratory. [Google Scholar]
- Hildebrandt H, Becker C, Murau M, Gerardy-Schahn R, Rahmann H. 1998. Heterogeneous expression of the polysialyltransferases ST8Sia II and ST8Sia IV during postnatal rat brain development. J Neurochem. 71:2339–2348. [DOI] [PubMed] [Google Scholar]
- Hildebrandt H, Mühlenhoff M, Weinhold B, Gerardy-Schahn R. 2007. Dissecting polysialic acid and NCAM functions in brain development. J Neurochem. 103:56–64. [DOI] [PubMed] [Google Scholar]
- Hildebrandt H, Mühlenhoff M, Oltmann-Norden I, Röckle I, Burkhardt H, Weinhold B, Gerardy-Schahn R. 2009. Imbalance of neural cell adhesion molecule and polysialyltransferase alleles causes defective brain connectivity. Brain. 132:2831–2838. [DOI] [PubMed] [Google Scholar]
- Hildebrandt H, Dityatev A. 2015. Polysialic acid in brain development and synaptic plasticity. Topics Current Chem. 366:55–96. [DOI] [PubMed] [Google Scholar]
- Hormuzdi SG, Filippov MA, Mitropoulou G, Monyer H, Bruzzone R. 2004. Electrical synapses: A dynamic signaling system that shapes the activity of neuronal networks. Biochim Biophys Acta. 1662:113–137. [DOI] [PubMed] [Google Scholar]
- Jakovcevski I, Mo Z, Zecevic N. 2007. Down-regulation of the axonal PSA-NCAM expression coincides with the onset of myelination in the human fetal forebrain. Neurosci. 149:328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CP, Fujimoto I, Rutishauser U, Leckband DE. 2005. Direct evidence that neural cell adhesion molecule (NCAM) polysialylation increases inter membrane repulsion and abrogates adhesion. J Biol Chem. 280:137–145. [DOI] [PubMed] [Google Scholar]
- Koutsoudaki PN, Hildebrandt H, Gudi V, Skripuletz T, Skuljec J, Strangel M. 2010. Remyelination after cuprizone-induced demyelination is accelerated in mice deficient in polisialic acid synthesizing enzyme ST8siaIV. Neurosci. 171:235–244. [DOI] [PubMed] [Google Scholar]
- Kuhn PL, Petroulakis E, Zazanis GA, McKinnon RD. 1995. Motor function analysis of myelin mutant mice using a rotarod. Internaltl J Dev Neurosci. 13:715–722. [DOI] [PubMed] [Google Scholar]
- Kurosawa N, Yoshida Y, Kojima N, Tsuji S. 1997. Polysialic acid synthase (ST8Sia II/STX) mRNA expression in the developing mouse central nervous system. J Neurochem. 69:494–503. [DOI] [PubMed] [Google Scholar]
- Lever C, Burton S, O’Keefe J. 2006. Rearings on hind legs, environmental novelty, and hippocampal formation. Rev Neurosci. 17:111–133. [DOI] [PubMed] [Google Scholar]
- Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. 1996. Chain migration of neuronal precursors. Science. 271:978–981. [DOI] [PubMed] [Google Scholar]
- Luzzati F, Peretto P, Aimar P, Ponti G, Fasolo A, Bonfanti L. 2003. Glia-independent chains of neuroblasts through the subcortical parenchyma of the adult rabbit brain. Proc Natl Acad Sci U S A. 100:13036–13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Franke A, Shen S, Barres BA. 1999. Astrocytes induce oligodendrocyte processes to alignwith and adhere to axons. Mol Cell Neurosci. 14:385–397. [DOI] [PubMed] [Google Scholar]
- Mikkonen M, Soininen H, Kalvianen R, Tapiola T, Ylinen A, Vapalahti M, Paljarvi L, Pitkanen A. 1998. Remodeling of neuronal circuitries in human temporal lobe epilepsy: Increased expression of highly polysialylated neural cell adhesion molecule in the hippocampus and the entorhinal cortex. Annals Neurol. 44:923–934. [DOI] [PubMed] [Google Scholar]
- Mikkonen M, Soininen H, Tapiola T, Alafuzoff I, Miettinen R. 1999. Hippocampal plasticity in Alzheimer’s disease: Changes in highly polysialylated NCAM immunoreactivity in the hippocampal formation. Eur J Neurosci. 11:1754–1764. [DOI] [PubMed] [Google Scholar]
- Mikkonen M, Soininen H, Alafuzof I, Miettinen R. 2001. Hippocampal plasticity in Alzheimer’s disease. Rev Neurosci. 12:311–325. [DOI] [PubMed] [Google Scholar]
- Miller RH. 2002. Regulation of oligodendrocyte development in the vertebrate CNS. Prog Neurobiol. 67:451–467. [DOI] [PubMed] [Google Scholar]
- Mitew S, Hay CM, Peckham H, Xiao J, Koenning M, Emery B. 2013. Mechanisms regulating the development of oligodendrocytes and central nervous system myelin. Neurosci. 12:29–47. [DOI] [PubMed] [Google Scholar]
- Mühlenhoff M, Eckhardt M, Bethe A, Frosch M, Gerardy-Schahn R. 1996. Autocatalytic polysialylation of polysialyltransferase-1. EMBO J. 15:6943–6950. [PMC free article] [PubMed] [Google Scholar]
- Mühlenhoff M, Eckhardt M, Gerardy-Schahn R. 1998. Polysialic acid: Three-dimensional structure, biosynthesis and function. Curr Opinion Structural Biol. 8:558–564. [DOI] [PubMed] [Google Scholar]
- Mühlenhoff M, Stummeyer K, Grove M, Sauerborn M, Gerardy-Schahn R. 2003. Proteolytic processing and oligomerization of bacteriophage-derived endosialidases. J Biol Chem. 278:12634–12644. [DOI] [PubMed] [Google Scholar]
- Mühlenhoff M, Rollenhagen M, Werneburg S, Gerardy-Schahn R, Hildebrandt H. 2013. Polysialicacid: Versatile modification of NCAM, SynCAM 1 and Neuropilin-2. Neurochem Res. 38:1134–1143. [DOI] [PubMed] [Google Scholar]
- Ojha J, Karmegam RV, Masilamoni JG, Terry AV Jr, Cashikar AG. 2011. Behavioral defects in chaperone-deficient alzheimer’s disease model mice. PLoS One. 6: e16550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltmann-Norden I, Galuska SP, Hildebrandt H, Geyer R, Gerardy-Schahn R, Gever H, Mühlenhoff M. 2008. Impact of the polysialyltransferases ST8SiaII and ST8SiaIV on polysialic acid synthesis during postnatal mouse brain development. J Biol Chem. 283:1463–1471. [DOI] [PubMed] [Google Scholar]
- Ong E, Nakayama J, Angata K, Reyes L, Katsuyama T, Arai Y, Fukuda M. 1998. Developmental regulation of polysialic acid synthesis in mouse directed by two polysialyltransferases, PST and STX. Glycobiology. 8:415–424. [DOI] [PubMed] [Google Scholar]
- Povlsen GK, Ditlevsen DK, Berezin V, Bock E. 2003. Intracellular signaling by the neural cell adhesion molecule. Neurochem Res. 28:127–141. [DOI] [PubMed] [Google Scholar]
- Probstmeier R, Bilz A, Schneider-Schaulies J. 1994. Expression of the neural cell adhesion molecule and polysialic acid during early mouse embryogenesis. J Neurosci Res. 37:324–335. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. 2003. The open field as a paradigm to measure the effects of drugs on anxiety likebehaviors: A review. Eur J Pharmacol. 463:3–33. [DOI] [PubMed] [Google Scholar]
- Rutishauser U, Landmesser L. 1996. Polysialic acid in the vertebrate nervous system: A promoter of plasticity in cell-cell interactions. Trends Neurosci. 19:422–427. [DOI] [PubMed] [Google Scholar]
- Rutishauser U. 2008. Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat Rev Neurosci. 9:26–35. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory. [Google Scholar]
- Schiff M, Weinhold B, Grothe C, Hildebrandt H. 2009. NCAM and polysialyltransferase profiles match dopaminergic marker gene expression but polysialic acid is dispensable for development of the midbrain dopamine system. J Neurochem. 110:1661–1673. [DOI] [PubMed] [Google Scholar]
- Schnaar RL, Gerardy-Schahn R, Hildebrandt H. 2014. Sialic acids in the brain: Gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol Rev. 94:461–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibenhener ML, Wooten MC. 2015. Use of the open field maze to measure locomotor and anxiety-like behavior in mice. J Vis Exp. (96):e52434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T, Arai Y. 1993. Distribution and possible roles of the highly polysialylated neural cell adhesion molecule (NCAM-H) in the developing and adult central nervous system. Neurosci Res. 17:265–290. [DOI] [PubMed] [Google Scholar]
- Seki T, Rutishauser U. 1998. Removal of polysialic acid-neural cell adhesion molecule induces aberrant mossy fiber innervation and ectopic synaptogenesis in the hippocampus. J Neurosci. 18:3757–3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford SC. 2007. The open field test: Reinventing the wheel. J Psychopharmacol. 21:134–135. [DOI] [PubMed] [Google Scholar]
- Stork O, Welzl H, Wotjak CT, Hoyer D, Delling M, Cremer H, Schachner M. 1999. Anxiety and increased 5-HT1A receptor response in NCAM null mutant mice. J Neurobiol. 40:343–355. [DOI] [PubMed] [Google Scholar]
- Szewczyk LM, Werneburg S, Brozko N, Hildebrandt H, Nagalski A, Wisniewska MB, Röckle I, Kuznicki J. 2017. ST8SIA2 promotes oligodendrocyte differentiation and the integrity of myelinand axons. Glia. 65:34–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Abeelen JHF. 1975. Genetic analysis of behavioural responses to novelty in mice. Nature. 254:239–241. [DOI] [PubMed] [Google Scholar]
- van der Putten H, Wiederhold KH, Probst A, Barbieri S, Mistl C, Danner S, Kauffmann S, Hofele K, Spooren WP, Ruegg MA et al. . 2000. Neuropathology in mice expressing human alpha-synuclein. J Neurosci. 20:6021–6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varea E, Guirado R, Gilabert-Juan J, Marti U, Castillo-Gomez E, Blasco-Ibanez JM, Crespo C, Nacher J. 2012. Expression of PSA-NCAM and synaptic proteins in the amygdala of psychiatric disorder patients. J Psychiatric Res. 46:189–197. [DOI] [PubMed] [Google Scholar]
- Walmod PS, Kolkova K, Berezin V, Bock E. 2004. Zippers make signals: NCAM-mediated molecular interactions and signal transduction. Neurochem Res. 29:2015–2035. [DOI] [PubMed] [Google Scholar]
- Walsh RN, Cummins RA. 1976. The open-field test: A critical review. Psychol Bull. 83:482–504. [PubMed] [Google Scholar]
- Wang Y, Pan Y, Price A, Martin LJ. 2011. Generation and characterization of transgenic mice expressing mitochondrial targeted red fluorescent protein selectively in neurons: Modeling mitochondriopathy in excitotoxicity and amyotrophic lateral sclerosis. Mol Neurodegen. 6:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhold B, Seidenfaden R, Röckle I, Mühlenhoff M, Schertzinger F, Conzelmann S, Marth JD, Gerardy-Schahn R, Hildebrandt H. 2005. Genetic ablation of polysialic acid causes severe neurodevelopmental defects rescued by deletion of the neural cell adhesion molecule. J Biol Chem. 280:42971–42977. [DOI] [PubMed] [Google Scholar]
- Werneburg S, Fuchs HLS, Albers I, Burkhardt H, Gudi V, Skripuletz T, Stangel M, Gerardy-Schahn R, Hildebrandt H. 2017. Polysialylation at early stages of oligodendrocyte differentiation promotes myelin repair. J Neurosci. 37:8131–8141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler Y, Benjamini Y, Golani I. 2018. Vertical exploration and dimensional modularity in mice. Royal Soc Open sci. 5: 180069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RC, Vacek T, Lanier DL, Dewsbury DA. 1976. Open-field behavior in muroid rodents. Behav Biol. 17:495–506. [DOI] [PubMed] [Google Scholar]
- Zhang H, Miller RH, Rutishauser U. 1992. Polysialic acid is required for optimal growth of axons on a neuronal substrate. J Neurosci. 12:3107–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zöller I, Büssow H, Gieselmann V, Eckhardt M. 2005. Oligodendrocyte-specific ceramidegalactosyltransferase (CGT) expression phenotypically rescues CGT-deficient mice and demonstrates that CGT activity does not limit brain galactosylceramide level. Glia. 52:190–198. [DOI] [PubMed] [Google Scholar]


