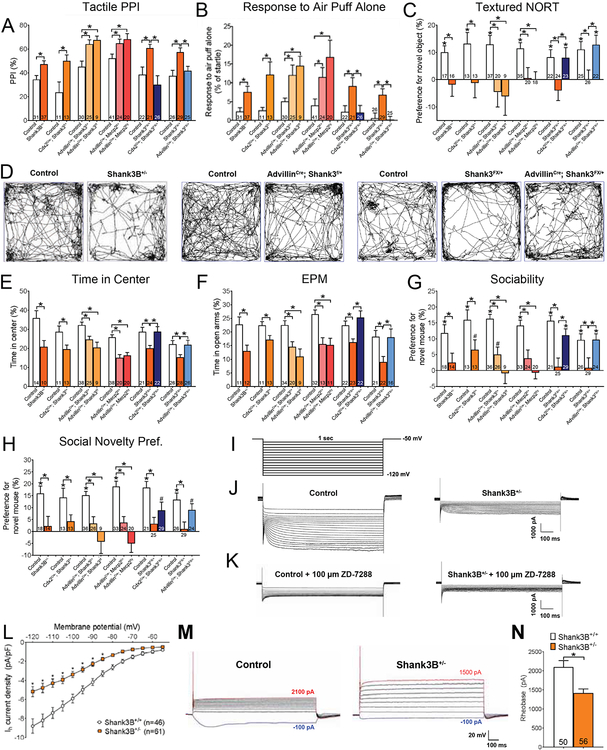
Figure 1. Shank3 functions cell-autonomously in peripheral somatosensory neurons for normal innocuous touch behaviors.
(A) Hairy skin sensitivity was measured using tactile PPI. Percent inhibition of the startle response to a 125 dB noise, when the startle noise is preceded by a light air puff (250 ms ISI). Student’s unpaired t-test or one-way ANOVA with post-hoc Tukey’s test, *, p < 0.05.
(B) Response to a light air puff stimulus alone directed to the back hairy skin. Responses are expressed as percent of startle response to a 125 dB noise. Student’s unpaired t-test or one-way ANOVA with post-hoc Tukey’s test, *, p < 0.05.
(C) Texture discrimination was measured using the textured NORT behavioral assay. A positive value indicates preference for the novel object, compared to the familiar object. Student’s unpaired t-test or one-way ANOVA with post-hoc Tukey’s test, *, p < 0.05.
(D) Open field (OF) test was used as a general measure of exploration and anxiety-like behavior. Shown are representative activity traces in the OF test for mutant mice and control littermates.
(E) Percent time spent in the center of the OF chamber. Student’s unpaired t-test or one-way ANOVA with post-hoc Tukey’s test, *, p < 0.05.
(F) Percent time spent in the open arms of the EPM. Student’s unpaired t-test or one-way ANOVA with post-hoc Tukey’s test, *, p < 0.05.
(G) Preference index for the percentage of time spent investigating a novel mouse, compared to a novel object, in the “Sociability” portion of the 3-chamber social interaction test. Student’s unpaired t-test or one-way ANOVA with post-hoc Tukey’s test, *, p < 0.05; #, p < 0.10.
(H) Preference index for the percentage of time spent investigating a novel mouse, compared to a familiar mouse, in the “Social Novelty Preference” portion of the 3-chamber social interaction test. Student’s unpaired t-test or one-way ANOVA with post-hoc Tukey’s test, *, p < 0.05; #, p < 0.10.
(I) Voltage step protocol used to activate HCN channels and elicit Ih during whole-cell voltage clamp recordings.
(J-K) Representative electrophysiological traces showing Ih during a hyperpolarizing voltage step protocol in large diameter DRG neurons cultured control and Shank3B+/− mutant mice, at baseline (K) and with a selective HCN-channel blocker, ZD-7288 (L).
(L) Quantification of Ih density at each voltage step for large diameter neurons cultured from DRGs of control and mutant mice. Two-way ANOVA with post-hoc Sidak’s test, [F (1,1470) =187.7; P < 0.0001] *, p < 0.05.
(M) Representative traces from large diameter DRG neurons cultured from control and Shank3B+/− mutant mice during whole cell current clamp recordings, in which the minimal amount of current required to elicit an action potential in each neuron (rheobase, Rh), was determined.
(N) Quantification of average R in large diameter DRG neurons cultured from control and Shank3B+/− mutant mice. Student’s unpaired t-test, *, p < 0.005.