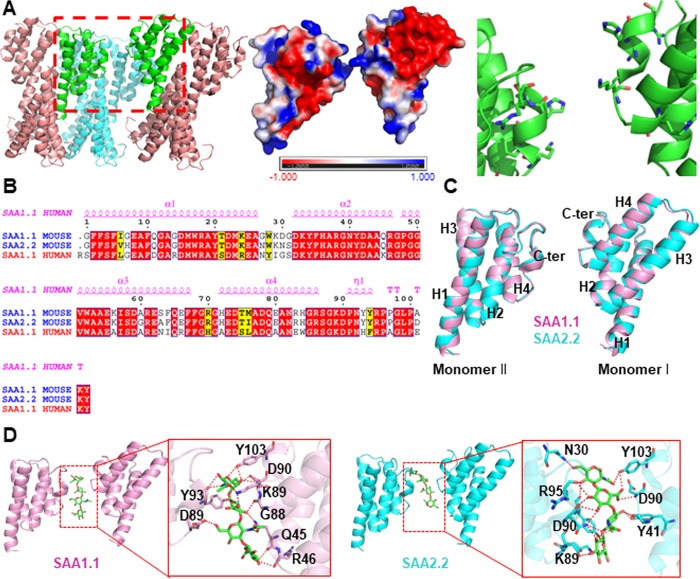
Figure 1.
Generation of a loosely bound dimeric model of mouse SAA1.1 and SAA2.2 dimers based on the human SAA1 crystal structure. (A) Three-dimensional structure of human SAA1 (PDB ID: 4IP8) dimer. Left-hand panel: human SAA1 oligomer constructed by asymmetric unit manipulation (the dimeric SAA1 model is highlighted in green). Center panel: surface charge distribution. Right-hand panel: interacting residues. (B) Sequence alignment of human SAA1 and murine SAA1.1 and SAA2.2 reveals high homology with more than 70% sequence identity. (C) Overlay of homology models of two interacting monomers of murine SAA1.1 (pink) and murine SAA2.2 (cyan) shows structural similarities between the SAA1.1 and SAA2.2 variants. (D) Hyaluronic acid (green sticks) binds to the cleft between two murine SAA monomers.