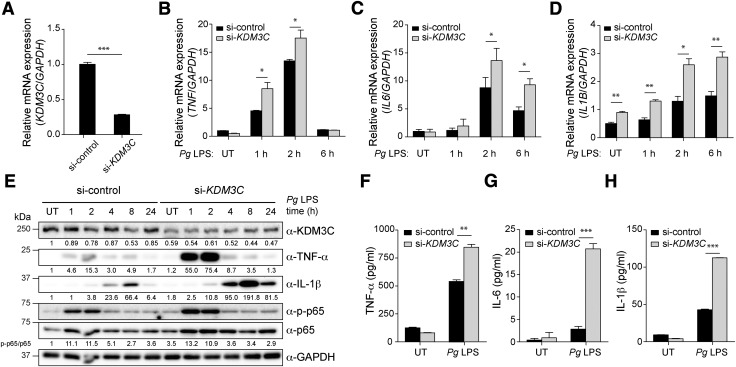
Figure 2.
KDM3C acts as a negative regulator of Pg LPS–induced proinflammatory signaling pathway in human THP-1 cells. A) si-KDM3C or si-control was transiently transfected into THP-1 cells. Real-time qPCR was used to measure endogenous KDM3C mRNA levels. B–D) Pg LPS–induced mRNA expressions of proinflammatory cytokines were assessed in THP-1 cells transfected with si-control or si-KDM3C by using real-time qPCR. Cells were harvested at the indicated times of Pg LPS (1 µg/ml) treatment. mRNA expressions of proinflammatory cytokines TNF (B), IL-6 (C), and IL-1B (D) were determined. E) Western blotting analysis of the levels of KDM3C, TNF-α, IL-1β, total p65 (NF-κB subunit), and phosphorylated p65 (p-p65) in control and KDM3C–knocked-down THP-1 cell treated with Pg LPS at indicated times. GAPDH was used as a loading control. The normalized intensities of the KDM3C, TNF-α, and IL-1β to GAPDH were calculated and shown as fold change below individual lanes. p-p65:p65 ratio was calculated and shown as fold change. These data are representative of at least 3 independent experiments. F–H) Cytokine levels in culture supernatants were measured by ELISA. THP-1 cells transfected with si-control or si-KDM3C were stimulated with Pg LPS (1 µg/ml) for 24 h. Secretions of cytokines, TNF-α (F), IL-6 (G), and IL-1β (H) were analyzed. UT, untreated. Data are representative of at least 3 independent experiments and statistically analyzed by Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001 compared with control cells (si-control).