Fig. 5.
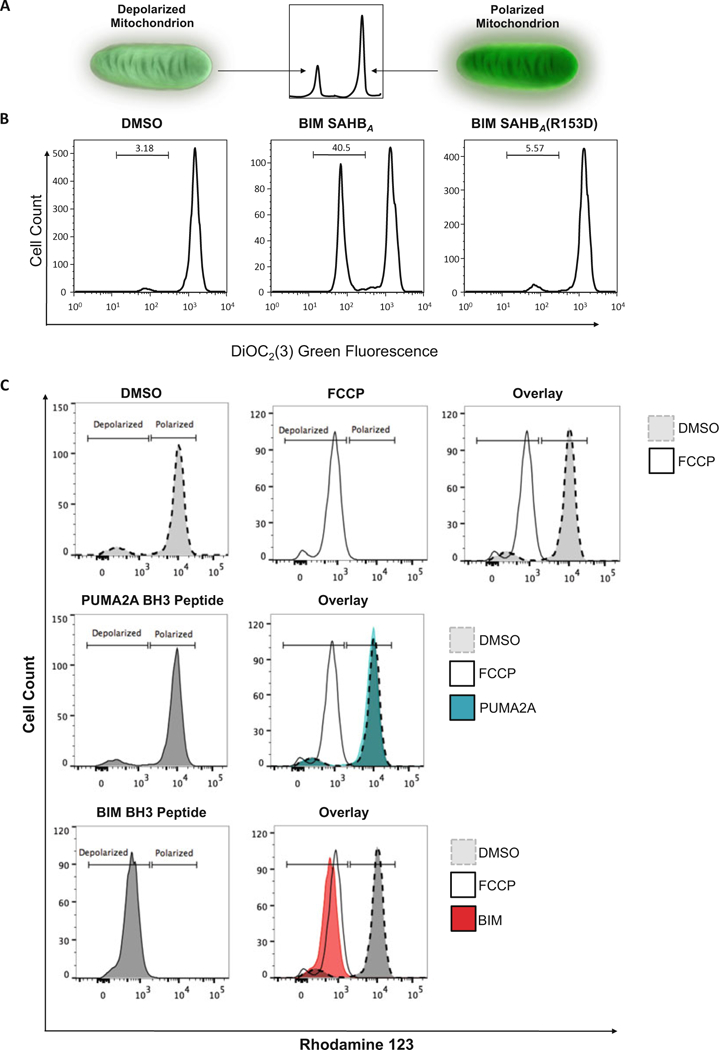
Measurement of mitochondrial outer membrane permeabilization (MOMP). (a) The general principle of how cationic dyes are used to measure MOMP is shown. As the membrane depolarizes and loses its negative charge, the cationic dyes will no longer associate with the mitochondria and the fluorescent signal will be lost. (b and c) Two examples of MOMP measurement are shown. (b) In the first example, MOMP is measured after treating cells with a stabilized alpha helix of BCL-2 domain (SAHB) modeled after the BH3-only protein BIM, which is known to induce apoptosis [30–33]. Treatment with BIM SAHBA (middle) causes partial membrane depolarization compared to DMSO (left) while treatment with a point mutant control, BIM SAHBA(R153D), results in no depolarization. (c) The second example demonstrates the use of BH3 peptides optimized to test the dependency of different cell types on specific anti-apoptotic proteins, a technique known as BH3 profiling [27, 34]. DMSO and FCCP are used as negative and positive controls respectively. These controls are overlaid and used as a reference guide in the measurement of the level of depolarization from other BH3 peptide treatments. The PUMA2A peptide serves as a negative control and does not cause membrane depolarization. BIM BH3 uniformly leads to complete depolarization in all cell types, and therefore overlaps with the FCCP treated sample
