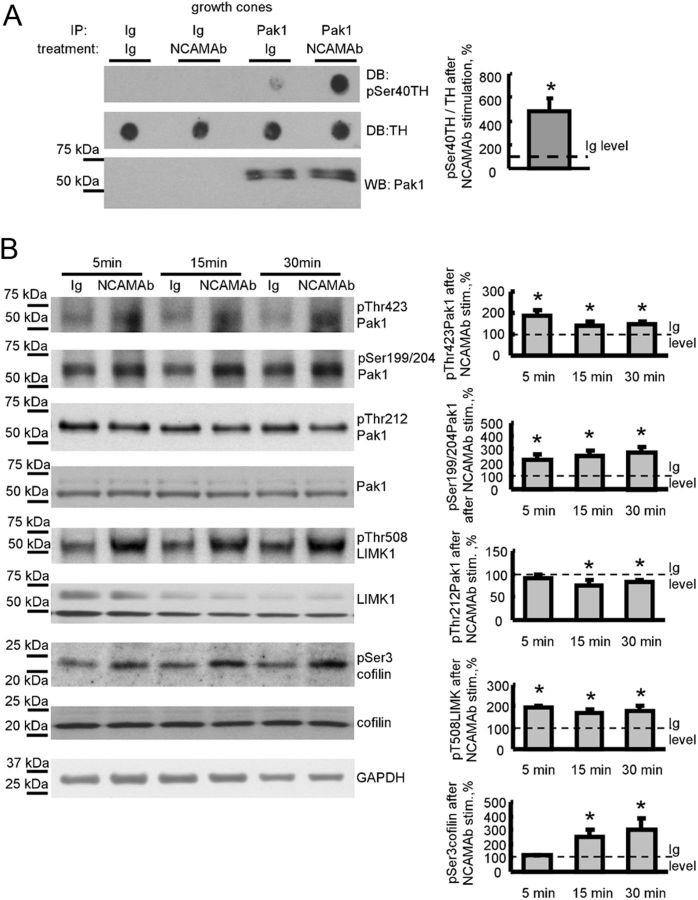
Figure 2.
NCAM activation activates the Pak1 signaling pathway in isolated growth cones. A, Growth cones isolated from brains of NCAM+/+ mice were pre-incubated with NCAM antibodies or nonimmune Ig (treatment). Pak1 was then immunoprecipitated from growth cone lysates (IP: Pak1). Mock immunoprecipitates with nonimmune Ig served as control (IP: Ig). Western blot (WB) with Pak1 antibodies shows that Pak1 was immunoprecipitated with the same efficiency from nonimmune Ig-treated and NCAM antibody-treated growth cones (bottom). The precipitates were incubated with TH peptide, and total levels of TH peptide and levels of TH peptide phosphorylated at Ser40 were analyzed by dot blot (DB). Note that Pak1 from NCAM antibody-treated growth cones phosphorylates TH peptide more efficiently than Pak1 from nonimmune Ig-treated growth cones. Graph shows quantitation of dot blots from three experiments. Mean + SEM values for NCAM antibody-treated probes normalized to the values from Ig-treated probes set to 100% (dashed line) are shown. *p < 0.05, paired t test. B, Growth cones isolated from NCAM+/+ mouse brains were incubated with polyclonal antibodies against the extracellular domain of NCAM for 5, 15, or 30 min. Growth cones treated with nonimmune Ig served as control. Western blots of the growth cone lysates probed with the indicated antibodies are shown. Graphs show quantitation of Western blots from five experiments. Mean + SEM values for NCAM antibody-treated growth cones normalized to the values from nonimmune Ig-treated growth cones set to 100% (dashed lines) are shown. Note that NCAM antibodies induce phosphorylation of Pak1 at Ser199/204 and Thr423, LIMK1 at Thr508, and cofilin at Ser3, and reduce phosphorylation of Pak1 at Thr212. Labeling for GAPDH was included as a loading control. *p < 0.05, paired t test, compared with corresponding probes from nonimmune Ig-treated growth cones.