Fig. 4. Gain-of-function of Cmpd-6 on the human β1AR mutants as compared to wild-type.
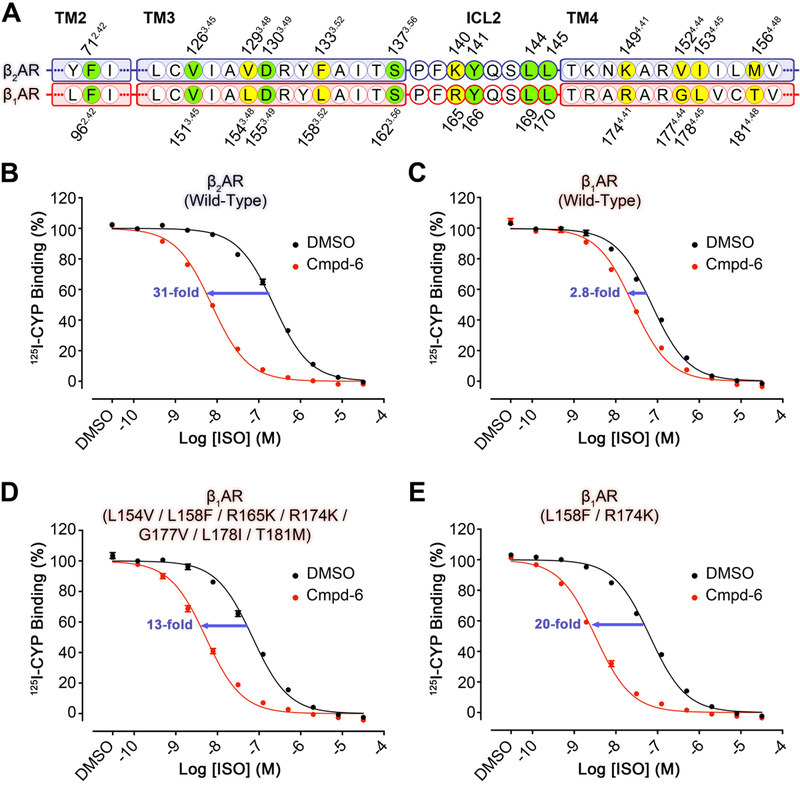
A, Partial protein sequence alignment of the human β2AR (blue highlight) and β1AR (red highlight) that includes only the relevant locations that engage Cmpd-6FA. Those residues that interact with Cmpd-6FA and are conserved are highlighted in green. Residues that are not conserved, but also interact with Cmpd-6FA are highlighted in yellow. Residue numbers for the β2AR and β1AR are shown above and below the alignment, respectively. B-E, Cmpd-6 positive allosteric effect on agonist binding of B, wild-type β2AR C, wild-type β1AR D, L154V / L158F / R165K / R174K / G177V / L178I / T181M β1AR mutant and E, L158F / R174K β1AR mutant. 125I-CYP vs. ISO competition binding was done using membrane preparations from HEK293 cells expressing the indicated receptor in the absence or presence of Cmpd-6 at 25 μM. Values were normalized to percentages of the maximal 125I-CYP binding level obtained from a one-site competition binding-Log IC50 curve fit (GraphPad Prism) of the vehicle (0.25% dimethylsulfoxide; DMSO) control data. Points on curves represent mean ± SEM obtained from four independent experiments performed in duplicate.
