Figure 7.
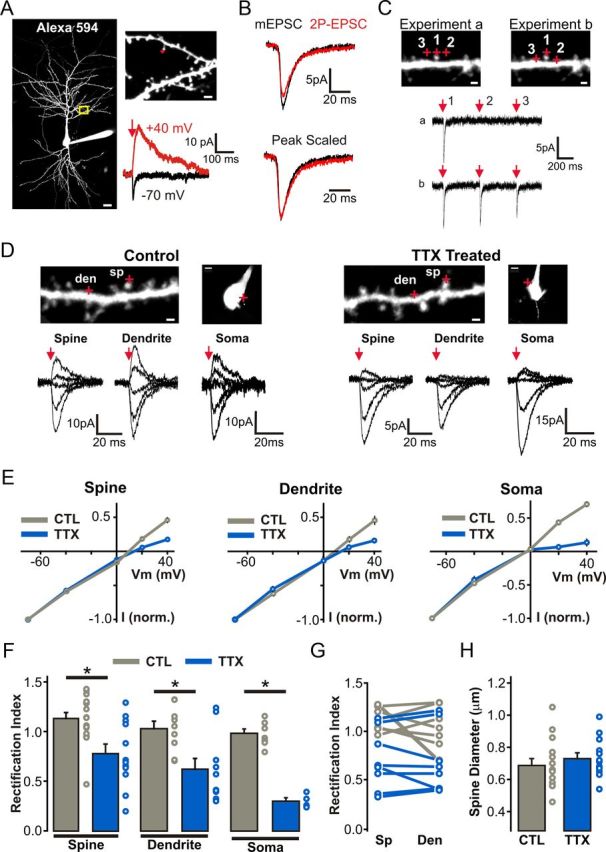
Cell-wide homeostatic upregulation of GluA2-lacking AMPARs in response to prolonged TTX treatment. A, Left, 2P image of a control CA1 pyramidal neuron filled with Alexa Fluor 594 to visualize dendritic morphology. Scale bar, 15 μm. Right, Enlarged view of an apical dendritic segment with red crosshairs illustrating the site of 2P glutamate uncaging (1 ms at 720 nm). Scale bar, 2 μm. At −70 mV, glutamate uncaging elicits a postsynaptic AMPAR-mediated response, whereas at +40 mV uncaging of glutamate also activates longer-decaying NMDAR EPSCs. B, Top, AMPAR-2P-EPSCs can be generated to match the amplitude of AMPAR-mEPSCs from the same recording. Bottom, Peak scaling of the average traces of AMPAR-2P-EPSCs and AMPAR-mEPSCs reveals a similar rise and decay time course. C, A set of control experiments whereby three uncaging pulses (separated by 500 ms) were elicited at each of the three points illustrated with red crosshairs. In experiment b, the uncaging positions of sites 2 and 3 were brought closer to the dendrite to elicit a response mediated by extrasynaptic receptors. Scale bars, 1 μm. D, I–V relationship of AMPAR-2P-EPSCs generated at distinct subcellular locations. Top, 2P images of secondary apical dendritic segment show sites of glutamate uncaging (red crosshairs). Scale bars: dendrite images, 1 μm; soma images, 5 μm. Bottom, AMPAR-2P-EPSCs at different holding potentials (−70 to +40 mV; with 100 μm intracellular spermine) with red arrow depicts the timing of the 1 ms uncaging pulse. E, Average I–V curves of 2P-EPSCs from each subcellular location in both control and TTX conditions. F, Rectification indices for all spine, dendritic, and somatic I–V curves presented in E (p < 0.01; unpaired Student's t test). G, Rectification indices of 2P-EPSCs generated from pairs of spine and neighboring (<5 μm) extrasynaptic shaft regions. H, Diameters (FWHM) of all dendritic spines probed for AMPAR-2P-EPSC I–V relationships.
